Question: What is the relationship between K, Kforward and Kreverse for a given reaction at equilibrium? A) K= Kreverse/Kforward B) K= Kforward/Kreverse C) 1/(Kforward)(Kreverse) D) Kforward=Kreverse
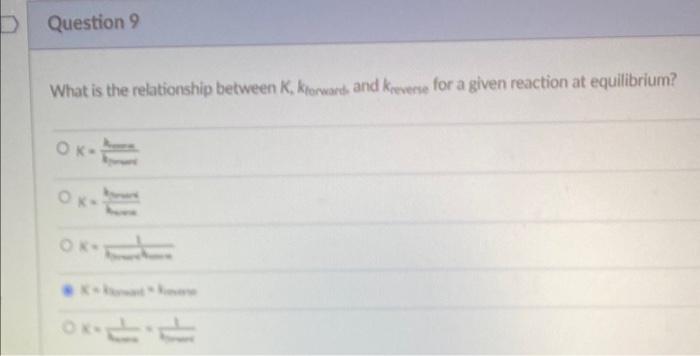
What is the relationship between K, Kforward and krevene for a given reaction at equilibrium? K=hsk X=menbsex K=knnx621 K* Bosate hinewe xman11+11
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


