Question: What is the volume that would be reported using the correct significant figures after the mathematical manipulation? 0.0167 mL -0.007523 mL = 0.009 mL 0.0092
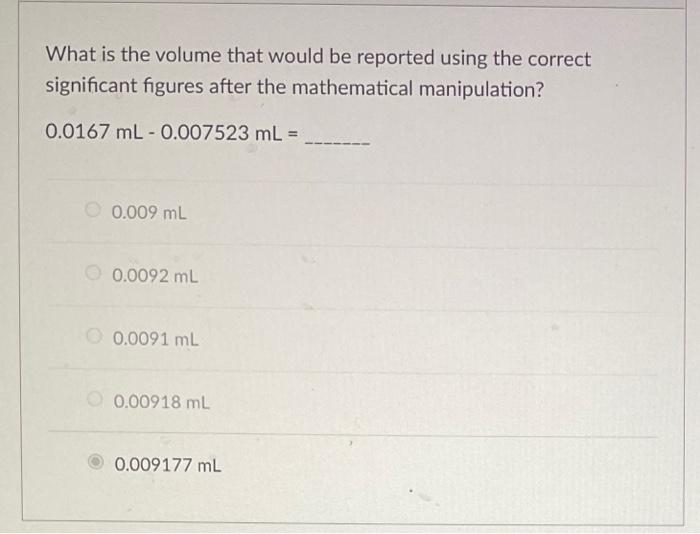
What is the volume that would be reported using the correct significant figures after the mathematical manipulation? 0.0167 mL -0.007523 mL = 0.009 mL 0.0092 mL 0.0091 mL 0.00918 mL 0.009177 ml
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


