Question: What is wrong with this solution? The irreversible liquid phase second order reaction (rA=kCA2) 2A4Bk=0.03dm3/mols is carried out in a CSTR. The entering concentration of
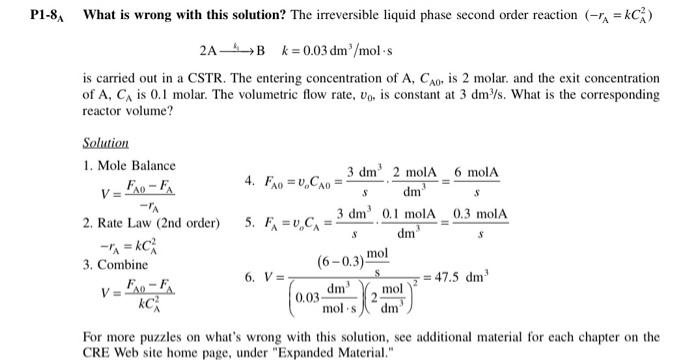
What is wrong with this solution? The irreversible liquid phase second order reaction (rA=kCA2) 2A4Bk=0.03dm3/mols is carried out in a CSTR. The entering concentration of A,CA0, is 2 molar. and the exit concentration of A, CA is 0.1 molar. The volumetric flow rate, v0, is constant at 3dm3/s. What is the corresponding reactor volume? Solution 1. Mole Balance V=rAFA0FA 4. FA0=voCA0=s3dm3dm32molA=s6molA 2. Rate Law (2nd order) 5. FA=voCA=s3dm3dm30.1molA=s0.3molA rA=kCA2 3. Combine V=kCA2FA0FA 6. V=(0.03molsdm3)(2dm3mol3)2(60.3)smol=47.5dm3 For more puzzles on what's wrong with this solution, see additional material for each chapter on the CRE Web site home page, under "Expanded Material
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


