Question: What mass of solid CO2 would be required to create 8757J of work at the normal sublimation temperature of 78.5C ? CO2(s)CO2(g)H=+26kJ
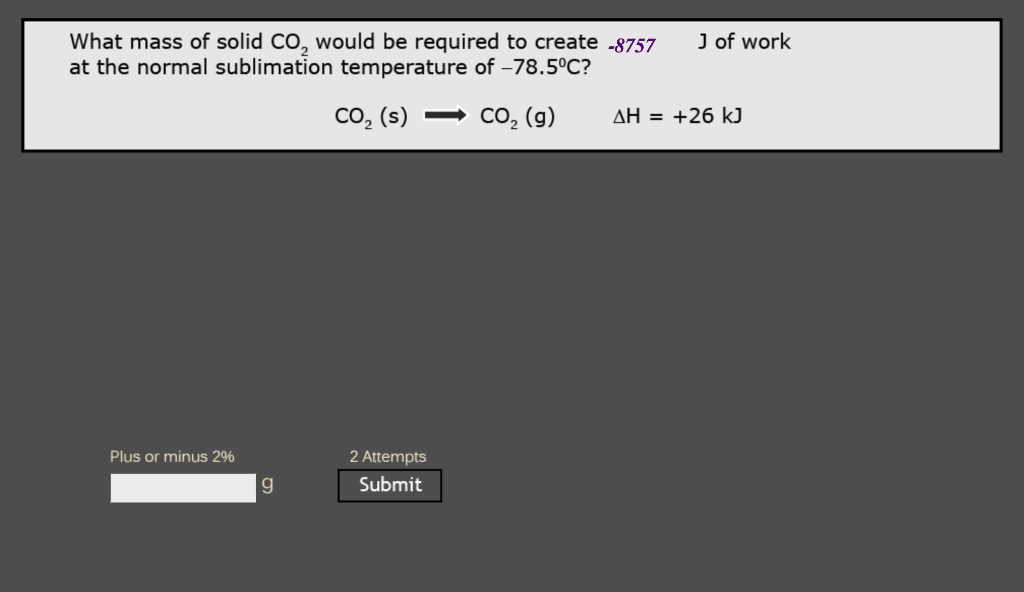
What mass of solid CO2 would be required to create 8757J of work at the normal sublimation temperature of 78.5C ? CO2(s)CO2(g)H=+26kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


