Question: whats the answer for this question ( the last answer wasnt smaller reaction order Consider the overall reaction: 2NO(g)+O2(g)2NO2(g) as well as the following data:
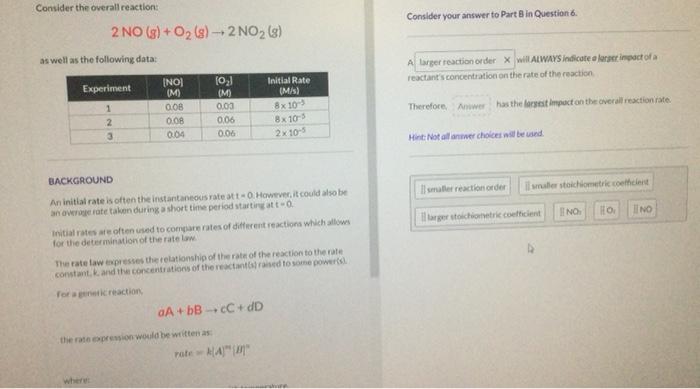
Consider the overall reaction: 2NO(g)+O2(g)2NO2(g) as well as the following data: Consider your answer to Part 8 in Question 6. as well as the following data: Therefore. has the larstat impucton the overall rexcioniate. Hint fiet allanmer choices aill be vaed. EACKGROUND An initlal rate is often the instantaneovis rate at t=0 Howtrec it could also be. an averoge rate taken during a short time period startires at t=0. Initial rates at often used to compare rates of dieferent reactiona which allows for the determination of the rate lam The fate taw expresses the relationship of tim rate of the resction to the rate. constant, k and the copcentrations of the rejctant(a)rased to soine power(s) fot a goine ki reaction; aA+bBcC+dD the rate eppespion would be witten as: rate=k(A)m{
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


