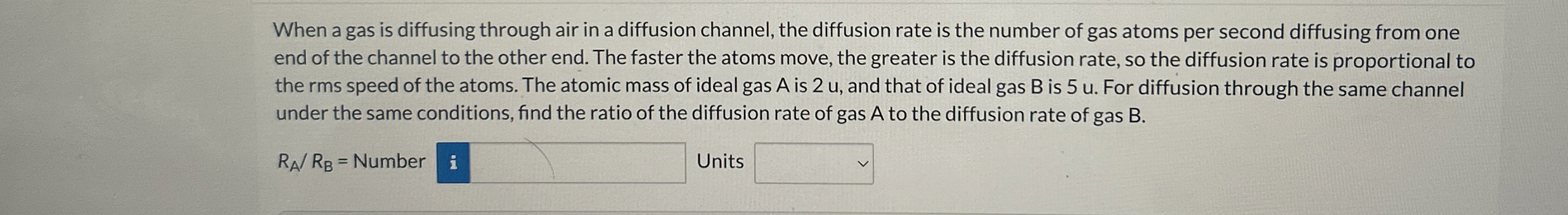
Question: When a gas is diffusing through air in a diffusion channel, the diffusion rate is the number of gas atoms per second diffusing from one
When a gas is diffusing through air in a diffusion channel, the diffusion rate is the number of gas atoms per second diffusing from one end of the channel to the other end. The faster the atoms move, the greater is the diffusion rate, so the diffusion rate is proportional to the rms speed of the atoms. The atomic mass of ideal gas is and that of ideal gas is For diffusion through the same channel under the same conditions, find the ratio of the diffusion rate of gas to the diffusion rate of gas
Number Units
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


