Question: When a pure metal crystallizes, it forms face-centered cubic cells. The unit cell edge length is 3.161A. The molar mass of the metal is 55.3913g/mol.
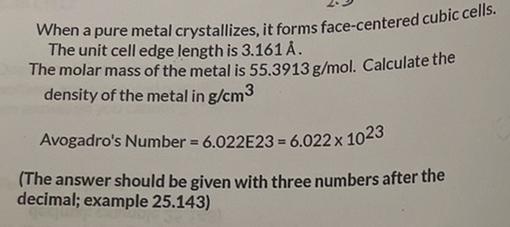
When a pure metal crystallizes, it forms face-centered cubic cells. The unit cell edge length is 3.161A. The molar mass of the metal is 55.3913g/mol. Calculate the density of the metal in g/cm3 Avogadro's Number =6.022E23=6.0221023 (The answer should be given with three numbers after the decimal; example 25.143)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


