Question: When a solid dissolves in water, heat may be evolved or absorbed. The heat of dissolution (dissolving) can be determined using a coffee cup calorimeter.
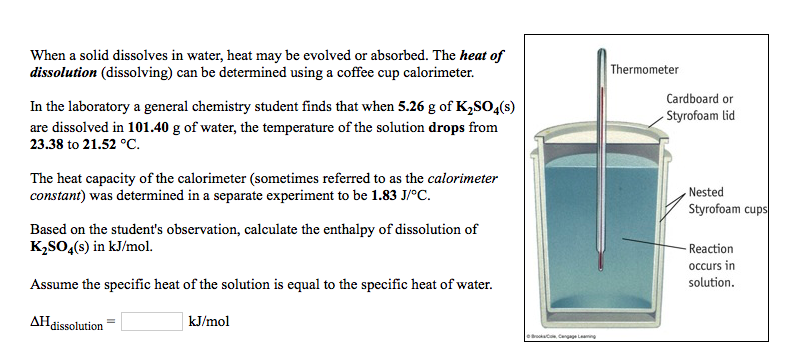
When a solid dissolves in water, heat may be evolved or absorbed. The heat of dissolution (dissolving) can be determined using a coffee cup calorimeter. Thermometer Cardboard or Styrofoam lid In the laboratory a general chemistry student finds that when 5.26 g of K2SO4(s) are dissolved in 101.40 g of water, the temperature of the solution drops from 23.38 to 21.52 C. The heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.83 J/C. Based on the student's observation, calculate the enthalpy of dissolution of K2SO4(s) in kJ/mol. Nested Styrofoam cups -Reaction occurs in solution. Assume the specific heat of the solution is equal to the specific heat of water. AH dissolution kJ/mol L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


