Question: When alkene 1 was treated with HBr, two alkyl halides (A and B) were isolated. a. With aid of a mechanism show how A and
When alkene 1 was treated with HBr, two alkyl halides (A and B) were isolated.
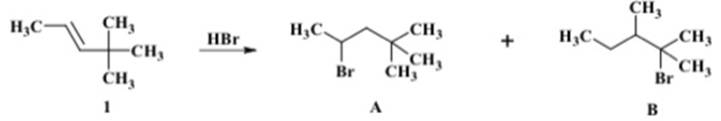
a. With aid of a mechanism show how A and B are formed under the above reaction conditions.
b. Which of two products isolated do you expect to be major? Give reasons.
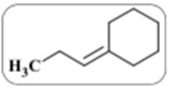
c. When the above compound is treated with BH 3 then NaOH and peroxide, what product s is/are obtained? Draw all structures and give IUPAC names.
H C- CH +CH, CHA 1 HBr H,C, Br A CH, SCH, CH3 H3C CH3 CH3 CH, Br B ,
Step by Step Solution
3.48 Rating (161 Votes )
There are 3 Steps involved in it
To address the questions a Mechanism for the Formation of A and B 1 Alkene Protonation The double bo... View full answer

Get step-by-step solutions from verified subject matter experts


