Question: Consider methanol synthesis from CO and H2, with a stoichiometric feed at pressures of 10, 30, and 100 atm. (a) If the reactor goes
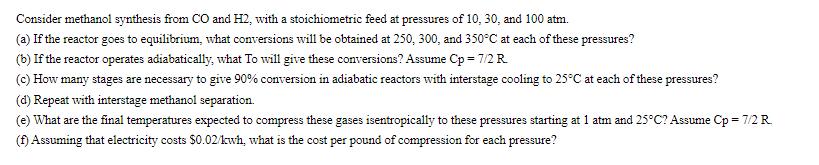
Consider methanol synthesis from CO and H2, with a stoichiometric feed at pressures of 10, 30, and 100 atm. (a) If the reactor goes to equilibrium, what conversions will be obtained at 250, 300, and 350C at each of these pressures? (b) If the reactor operates adiabatically, what To will give these conversions? Assume Cp = 7/2 R (c) How many stages are necessary to give 90% conversion in adiabatic reactors with interstage cooling to 25C at each of these pressures? (d) Repeat with interstage methanol separation. (e) What are the final temperatures expected to compress these gases isentropically to these pressures starting at 1 atm and 25C? Assume Cp = 7/2 R (f) Assuming that electricity costs $0.02/kwh, what is the cost per pound of compression for each pressure?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


