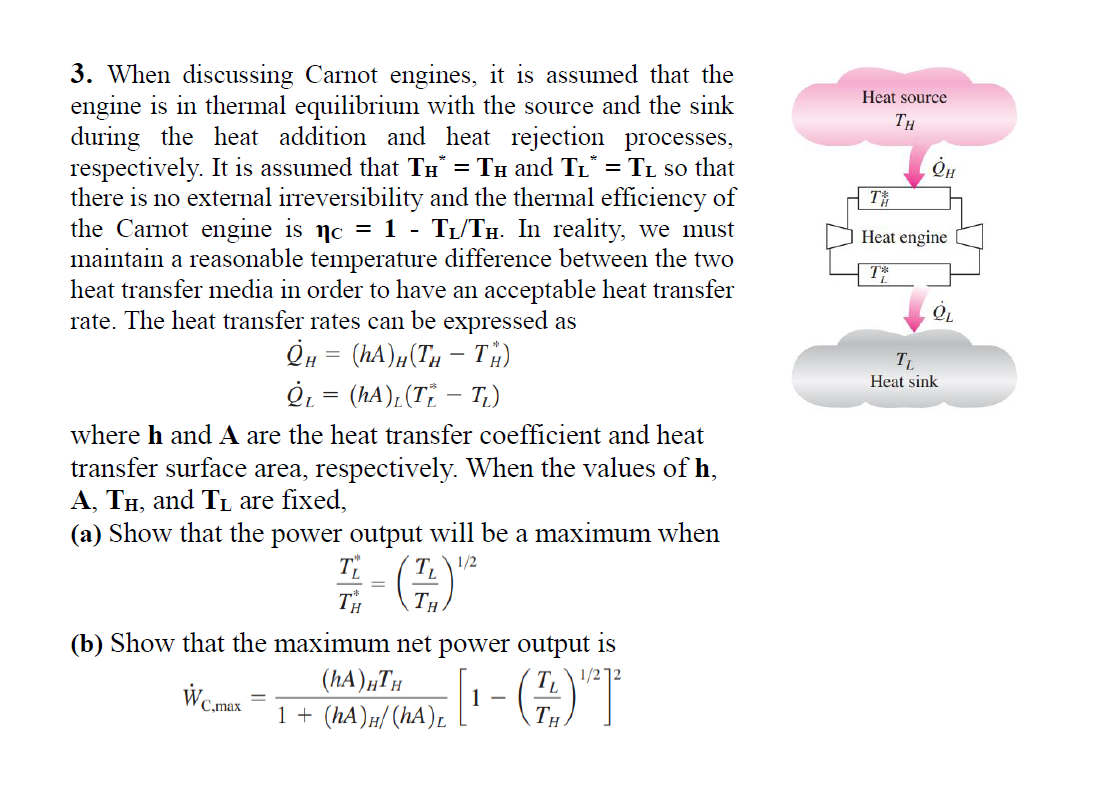
Question: When discussing Carnot engines, it is assumed that the engine is in thermal equilibrium with the source and the sink during the heat addition and
When discussing Carnot engines, it is assumed that the
engine is in thermal equilibrium with the source and the sink
during the heat addition and heat rejection processes,
respectively. It is assumed that and so that
there is no external irreversibility and the thermal efficiency of
the Carnot engine is In reality, we must
maintain a reasonable temperature difference between the two
heat transfer media in order to have an acceptable heat transfer
rate. The heat transfer rates can be expressed as
where and A are the heat transfer coefficient and heat
transfer surface area, respectively. When the values of
and are fixed,
a Show that the power output will be a maximum when
b Show that the maximum net power output is
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


