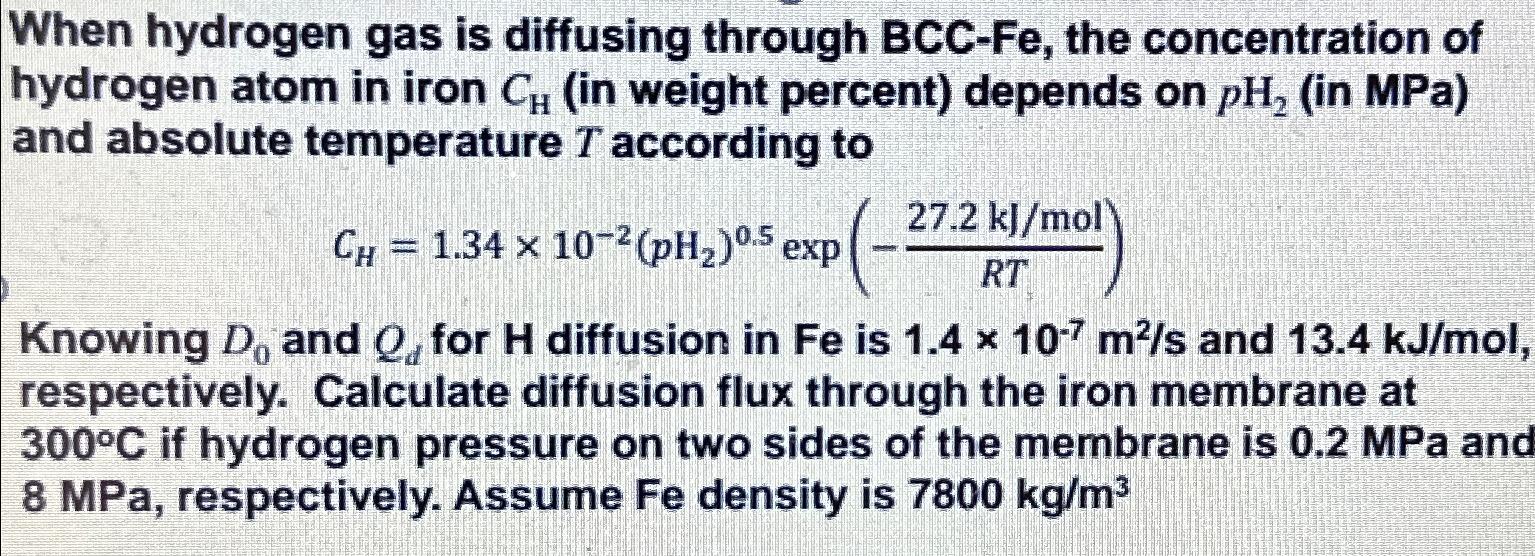
Question: When hydrogen gas is diffusing through B C C - Fe , the concentration of hydrogen atom in iron C H ( in weight percent
When hydrogen gas is diffusing through Fe the concentration of hydrogen atom in iron in weight percent depends on in MPa and absolute temperature according to
exp
Knowing and for diffusion in is and respectively. Calculate diffusion flux through the iron membrane at if hydrogen pressure on two sides of the membrane is MPa and MPa, respectively. Assume Fe density is
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


