Question: When Iron and steam react at high temperatures, the following reaction takes place 3 Fe(s) + 4 H, 0(9) FeOc(s) + 4 H (9) How
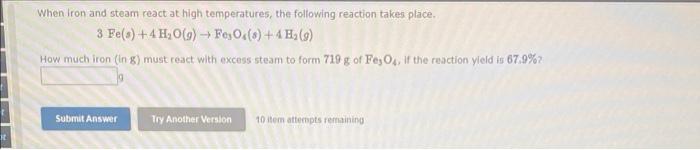
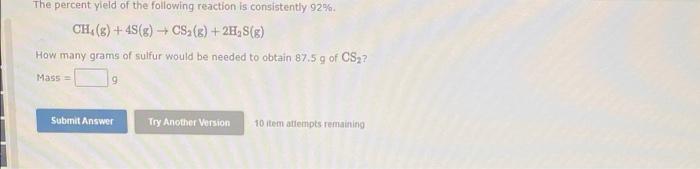

When Iron and steam react at high temperatures, the following reaction takes place 3 Fe(s) + 4 H, 0(9) FeOc(s) + 4 H (9) How much iron (In 8) must react with excess steam to form 719 g of Fe, O, if the reaction yield is 67.9% Submit Answer Try Another Version 10 item attempts remaining The percent yield of the following reaction is consistently 92% CH() +43(g) + CS2 (B) + 2H,S() How many grams of sulfur would be needed to obtain 87.5 g of CS2? Mass 9 Submit Answer Try Another Version 10 item attempts remaining References What volume of 0.756 M HCl, in milliliters, is required to titrate 2.70 g of NaOH to the equivalence point? NaOH(aq) + HCl(aq) + H2O() + NaCl(aq) - Volume ml Submit Answer Try Another Version 10 item attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


