Question: When magnesium reacts with fluorine to form an ionic compound, each metal atom loses electron(s) and each nonmetal atom gains electron(s). There must be magnesium
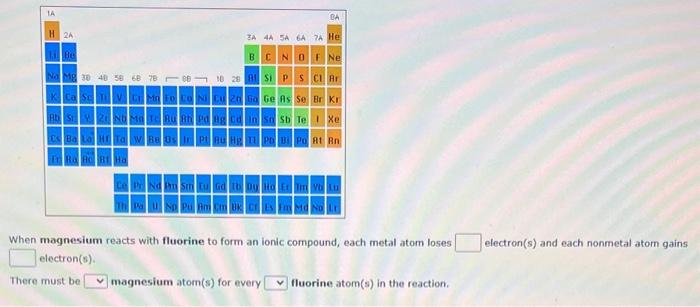
When magnesium reacts with fluorine to form an ionic compound, each metal atom loses electron(s) and each nonmetal atom gains electron(s). There must be magnesium atom(s) for every fluorine atom(s) in the reaction
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


