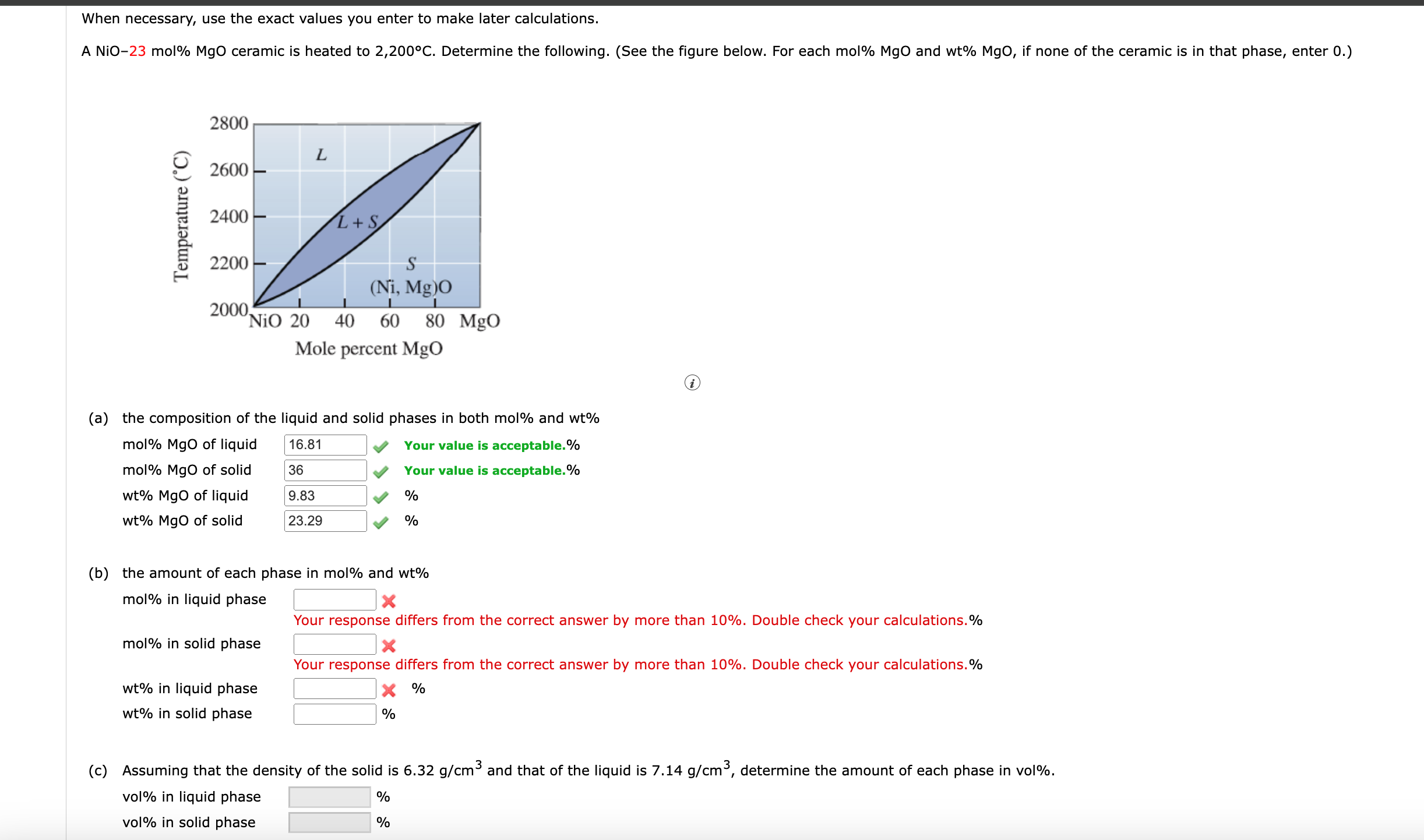
Question: When necessary, use the exact values you enter to make later calculations. ( a ) the composition of the liquid and solid phases in both
When necessary, use the exact values you enter to make later calculations.
a the composition of the liquid and solid phases in both mol and
molMgO of liquid Your value is acceptable.
molMgO of solid
Your value is acceptable.
MgO of liquid
MgO of solid
b the amount of each phase in mol and
mol in liquid phase
Your response differs from the correct answer by more than Double check your calculations.
mol in solid phase
Your response differs from the correct answer by more than Double check your calculations.
wt in liquid phase
in solid phase
times
c Assuming that the density of the solid is and that of the liquid is determine the amount of each phase in vol
vol in liquid phase
vol in solid phase
I dont understand how i am getting it wrong
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


