Question: When solid ammonium carbamate sublimes, it dissociates completely into ammonia and carbon dioxide according to the following equation: (NH4)(H2NCO2)(s)2NH3(g)+CO2(g) At 30C, experiment shows that the
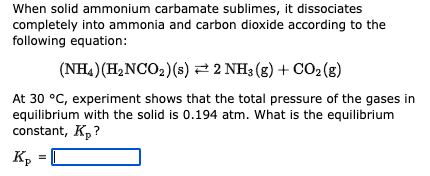
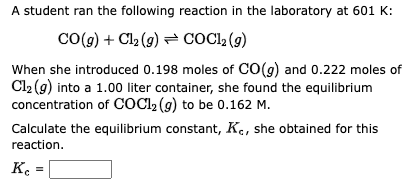
When solid ammonium carbamate sublimes, it dissociates completely into ammonia and carbon dioxide according to the following equation: (NH4)(H2NCO2)(s)2NH3(g)+CO2(g) At 30C, experiment shows that the total pressure of the gases in equilibrium with the solid is 0.194 atm. What is the equilibrium constant, Kp ? A student ran the following reaction in the laboratory at 601K : CO(g)+Cl2(g)COCl2(g) When she introduced 0.198 moles of CO(g) and 0.222 moles of Cl2(g) into a 1.00 liter container, she found the equilibrium concentration of COCl2(g) to be 0.162M. Calculate the equilibrium constant, Kc, she obtained for this reaction
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


