Question: When the following equations are balanced using the smallest possible integers, what will be the coefficient for the underlined substance in each case? Mg(s) +
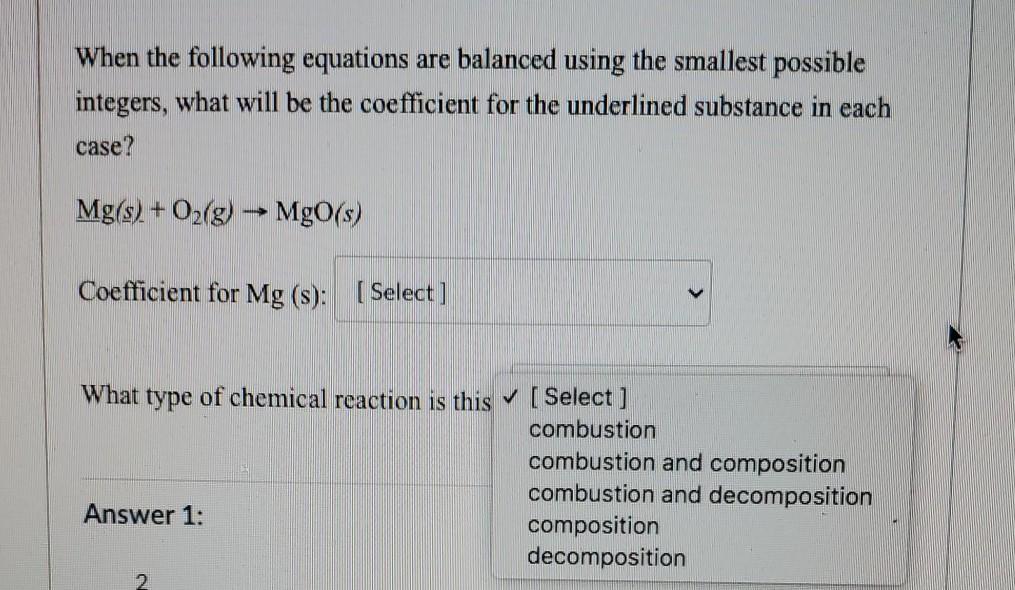
When the following equations are balanced using the smallest possible integers, what will be the coefficient for the underlined substance in each case? Mg(s) + O2(g) MgO(s) Coefficient for Mg(s): Select] What type of chemical reaction is this [Select ] combustion combustion and composition combustion and decomposition Answer 1: composition decomposition
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


