Question: When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: chlorine(g)+water(1)hydrochloricacid(aq)+ When the following molecular equation


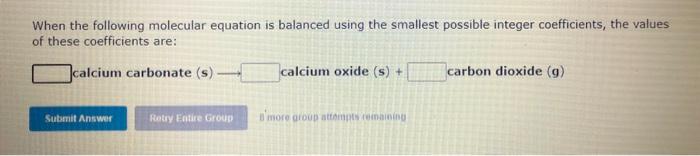
When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: chlorine(g)+water(1)hydrochloricacid(aq)+ When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: nitrogen(g)+hydrogen(g)ammonia(g) 8 more group atidmpts rumainga When the following molecular equation is balanced using the smallest possible integer coefficients, the values of these coefficients are: calciumcarbonate(s)calciumoxide(s)+carbondioxide(g) dimore group attongts i einaning
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


