Question: When toluene is treated with bromine the bromine does react with the double bond to form a viscinal dihalide, but instead a substitution of one
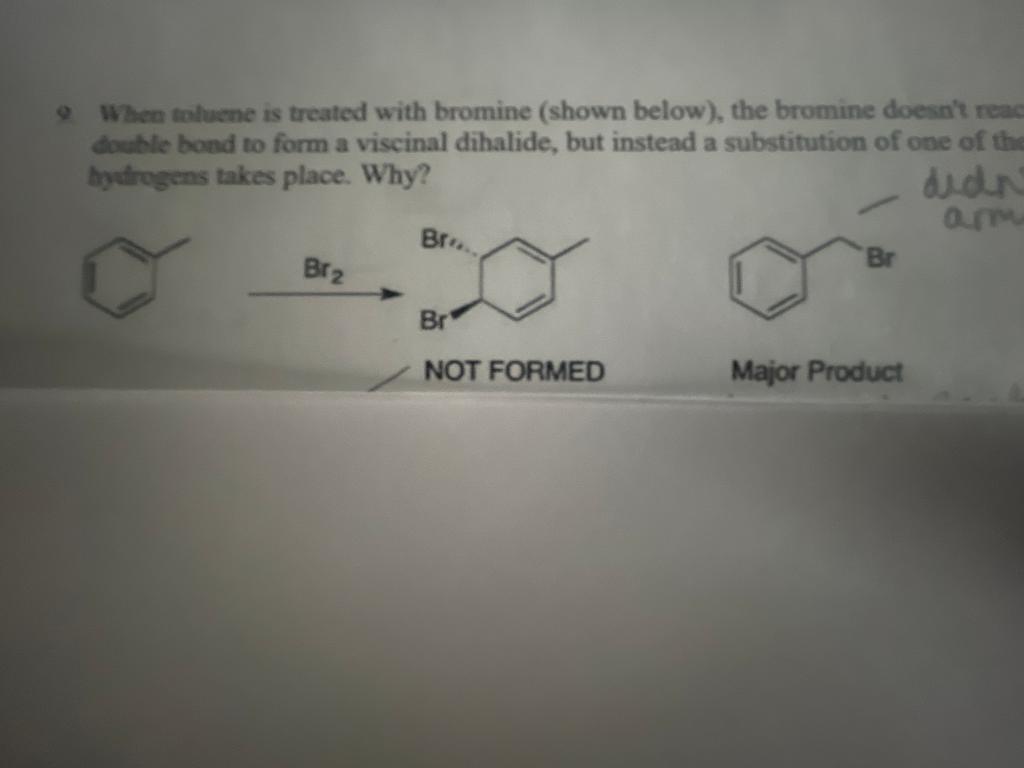 When toluene is treated with bromine the bromine does react with the double bond to form a viscinal dihalide, but instead a substitution of one of the benzylic hydrogens takes places. Why?
When toluene is treated with bromine the bromine does react with the double bond to form a viscinal dihalide, but instead a substitution of one of the benzylic hydrogens takes places. Why?
When tolucne is treated with bromine (shown below), the bromine doesn't read doable bond to form a viscinal dihalide, but instead a substitution of one of the bydrogens takes place. Why? NOT FORMED Major Product
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


