Question: When you are using NaBH4 or L-selectride, is it possible you can know which product dominates? In other words, does NaBH4 form the more dominant
 When you are using NaBH4 or L-selectride, is it possible you can know which product dominates? In other words, does NaBH4 form the more dominant trans product or does L-selectride form the more dominant trans product??
When you are using NaBH4 or L-selectride, is it possible you can know which product dominates? In other words, does NaBH4 form the more dominant trans product or does L-selectride form the more dominant trans product??
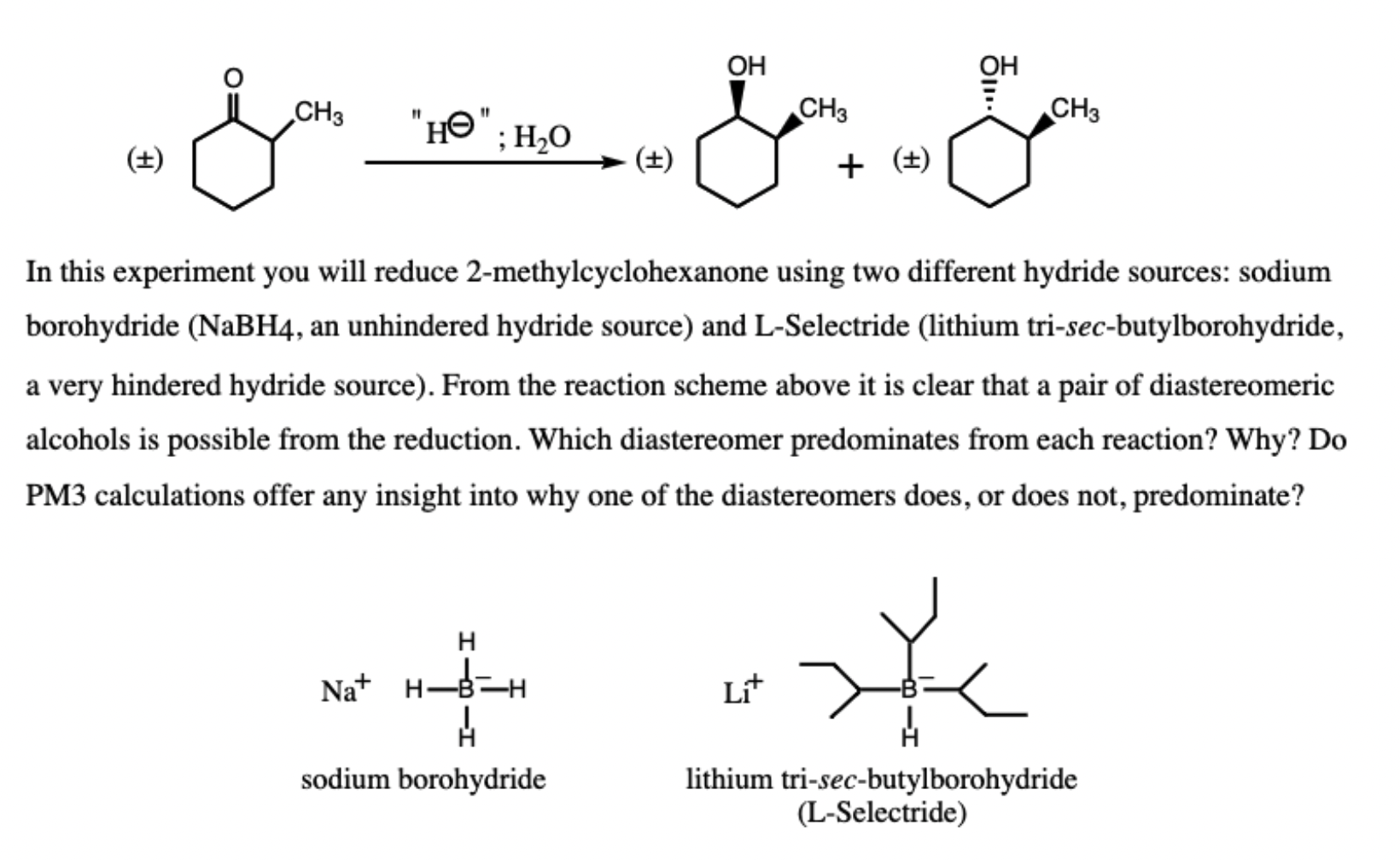
OH OH CH3 CH3 CH3 "HO": 1,0 (+) (+) + () In this experiment you will reduce 2-methylcyclohexanone using two different hydride sources: sodium borohydride (NaBH4, an unhindered hydride source) and L-Selectride (lithium tri-sec-butylborohydride, a very hindered hydride source). From the reaction scheme above it is clear that a pair of diastereomeric alcohols is possible from the reduction. Which diastereomer predominates from each reaction? Why? Do PM3 calculations offer any insight into why one of the diastereomers does, or does not, predominate? H Nat H-B= H Lit sodium borohydride lithium tri-sec-butylborohydride (L-Selectride)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


