Question: where am I going wrong in my calculations for question 1? I am doing the same steps as proceeded for question 2 and question 2
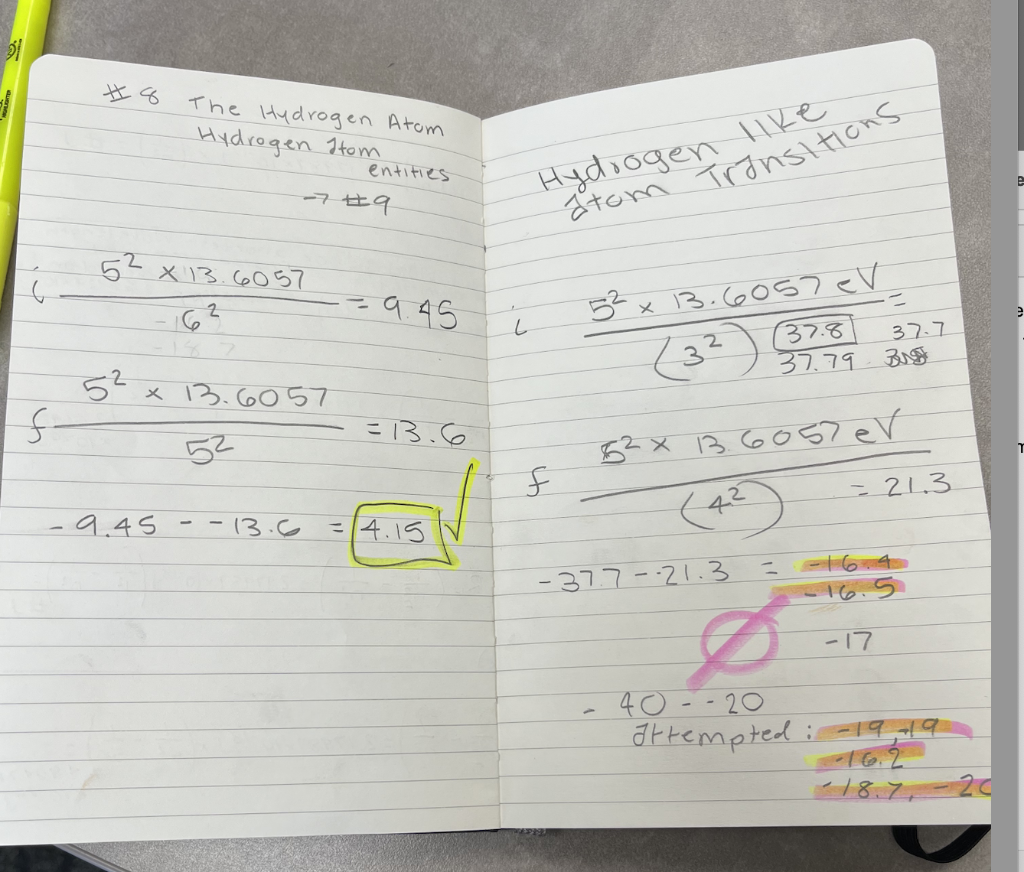 where am I going wrong in my calculations for question 1? I am doing the same steps as proceeded for question 2 and question 2 I have correct. For question 1 answers I have attempted and been told are incorrect are -16.4, -16.5, -17, -19, 19, -16.2, 18.7, 20, -20
where am I going wrong in my calculations for question 1? I am doing the same steps as proceeded for question 2 and question 2 I have correct. For question 1 answers I have attempted and been told are incorrect are -16.4, -16.5, -17, -19, 19, -16.2, 18.7, 20, -20
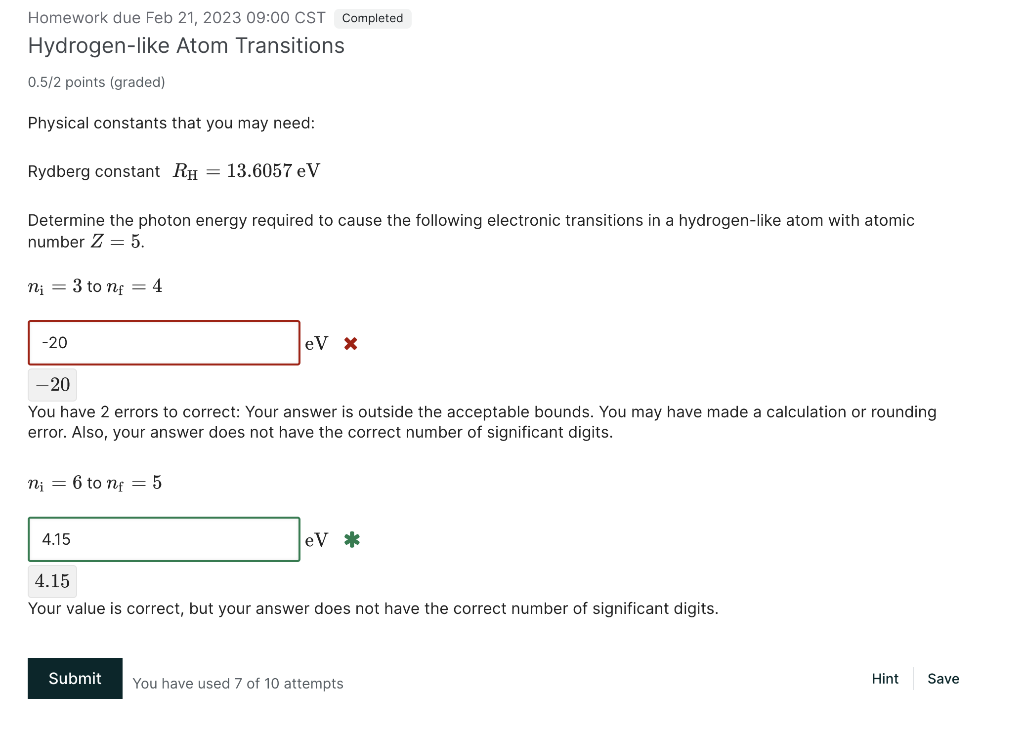
Homework due Feb 21, 2023 09:00 CST Hydrogen-like Atom Transitions 0.5/2 points (graded) Physical constants that you may need: Rydberg constant RH=13.6057eV Determine the photon energy required to cause the following electronic transitions in a hydrogen-like atom with atomic number Z=5. ni=3tonf=4 20 You have 2 errors to correct: Your answer is outside the acceptable bounds. You may have made a calculation or rounding error. Also, your answer does not have the correct number of significant digits. ni=6tonf=5 4.15 Your value is correct, but your answer does not have the correct number of significant digits. You have used 7 of 10 attempts \#8 The Hydrogen Atom Hydrogen 1tom entities Hydrogen Ilte jtom i625213.6057=9.45;(32)37.7937.8378.5213.6057eV=f525213.6057=13.69.4513.6=4.15(42)=21.252136057eV
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


