Question: where am I going wrong in my calculations? Quantum dot emmisions Physical constants that you may need: Speed of light c=2.99792108sm Planck's constant h=6.626071034Js Electron
 where am I going wrong in my calculations?
where am I going wrong in my calculations?
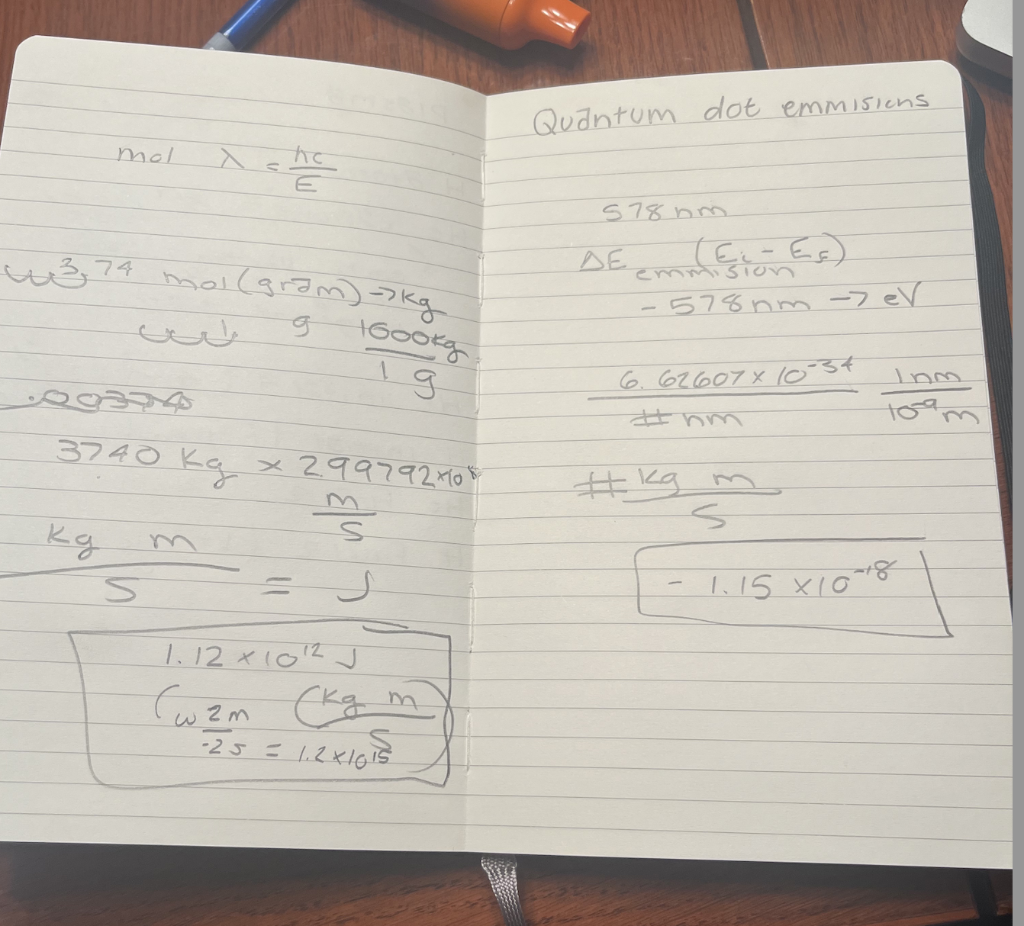
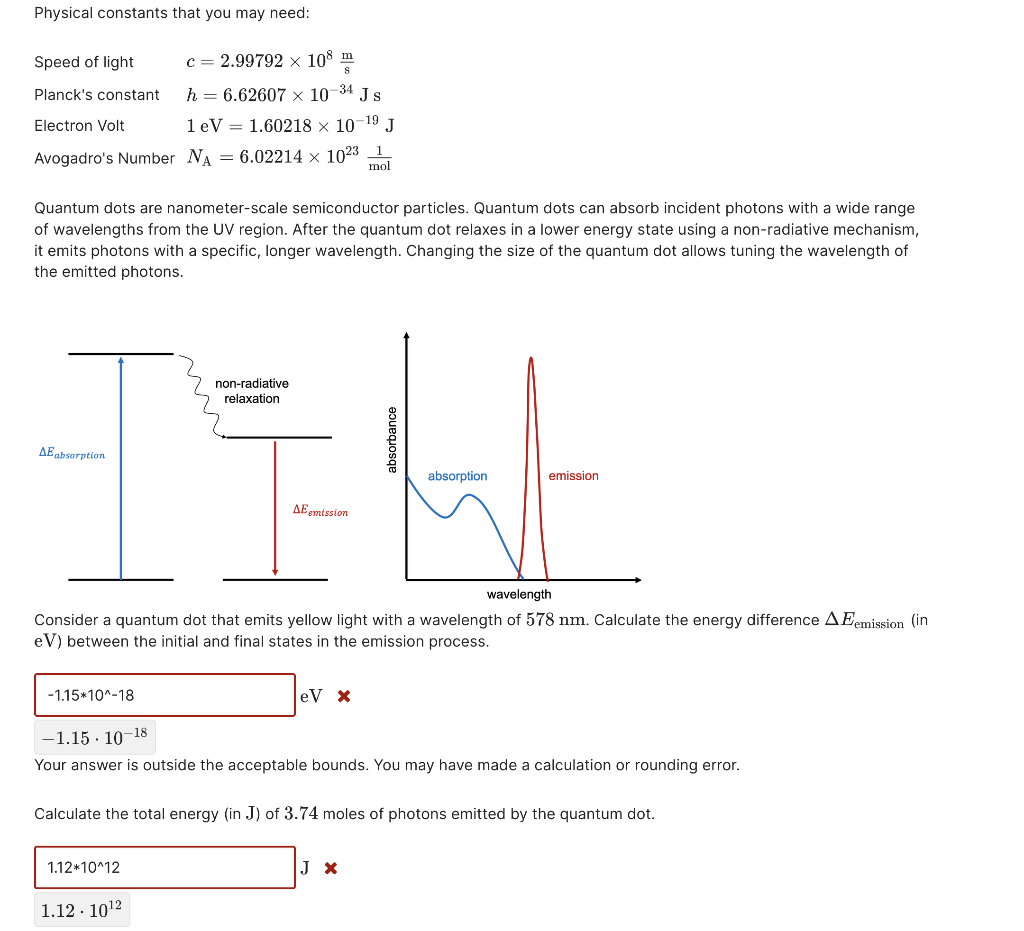
Quantum dot emmisions Physical constants that you may need: Speed of light c=2.99792108sm Planck's constant h=6.626071034Js Electron Volt 1eV=1.602181019J Avogadro's Number NA=6.022141023mol1 Quantum dots are nanometer-scale semiconductor particles. Quantum dots can absorb incident photons with a wide range of wavelengths from the UV region. After the quantum dot relaxes in a lower energy state using a non-radiative mechanism, it emits photons with a specific, longer wavelength. Changing the size of the quantum dot allows tuning the wavelength of the emitted photons. Consider a quantum dot that emits yellow light with a wavelength of 578nm. Calculate the energy difference Eemission (in eV ) between the initial and final states in the emission process. eVx
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


