Question: Which compound does not contain both polar covalent and ionic bonds? KOH O CH;OH O CsC,H3O2 O Ca(CN)2 O NHCIO,
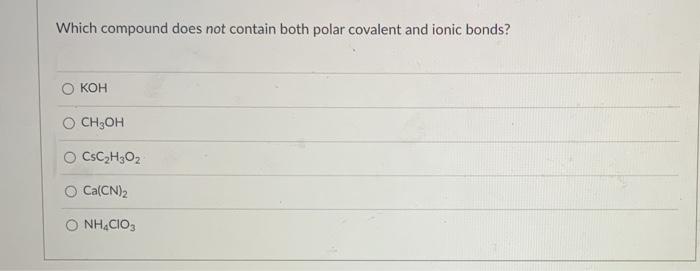
Which compound does not contain both polar covalent and ionic bonds? KOH O CH;OH O CsC,H3O2 O Ca(CN)2 O NHCIO,
Step by Step Solution
3.51 Rating (154 Votes )
There are 3 Steps involved in it
CH3OH has only covalent bonds While the ot... View full answer

Get step-by-step solutions from verified subject matter experts


