Question: Which equation best represents the net ionic equation for the reaction that occurs when aqueous solutions of sodium phosphate and magnesium nitrate are mixed? 3Mg2+(aq)+2PO43(aq)Mg3(PO4)2(aq)2Na3PO4(aq)+3Mg(NO3)2(aq)Mg3(PO4)2(s)+6NaNO3(aq)3Mg2+(aq)+2PO43(aq)Mg3(PO4)2(s)2Na+(aq)+Mg(NO3)2(aq)2NaNO3(aq)+Mg2+(aq)2Na3PO4(aq)+3Mg2+(aq)Mg3(PO4)2(s)+6Na+(aq)
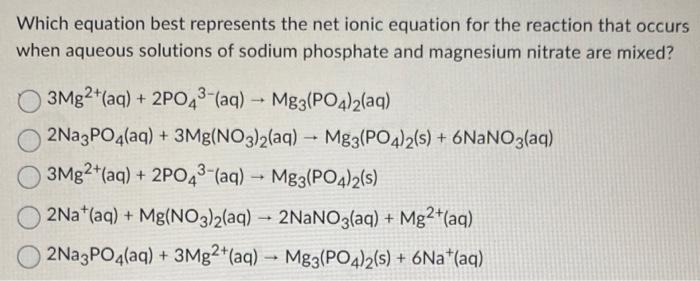
Which equation best represents the net ionic equation for the reaction that occurs when aqueous solutions of sodium phosphate and magnesium nitrate are mixed? 3Mg2+(aq)+2PO43(aq)Mg3(PO4)2(aq)2Na3PO4(aq)+3Mg(NO3)2(aq)Mg3(PO4)2(s)+6NaNO3(aq)3Mg2+(aq)+2PO43(aq)Mg3(PO4)2(s)2Na+(aq)+Mg(NO3)2(aq)2NaNO3(aq)+Mg2+(aq)2Na3PO4(aq)+3Mg2+(aq)Mg3(PO4)2(s)+6Na+(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


