Question: Which intermolecular force is responsible for the apparently low boiling points observed for alkanes when compared to polar molecules of similar molecular weight? Dipole -
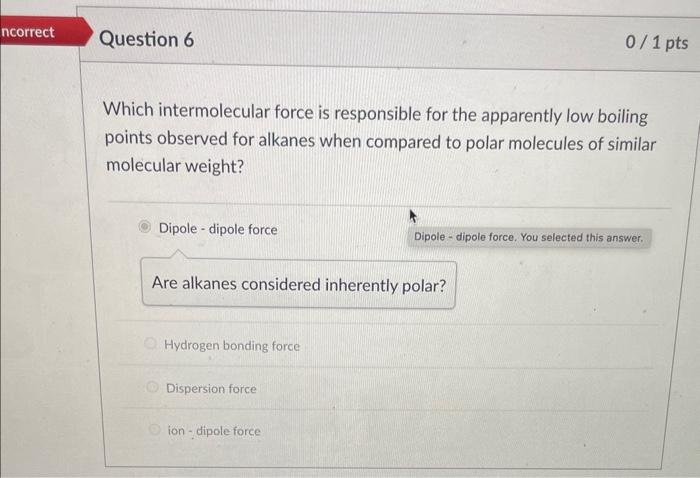
Which intermolecular force is responsible for the apparently low boiling points observed for alkanes when compared to polar molecules of similar molecular weight? Dipole - dipole force Are alkanes considered inherently polar? Hydrogen bonding force Dispersion force ion-dipole force
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


