Question: Which is the most complete charge balance expression for a saturated solution of CaC2O4 ? [Ca2+]=[C2O42]2[Ca2+]=2[C2O42]+[HC2O4][H+]+2[Ca2+]=[OH]+2[C2O42][H+]+2[Ca2+]=[OH]+2[C2O42]+[HC2O4] How much mental effort did you expend on this
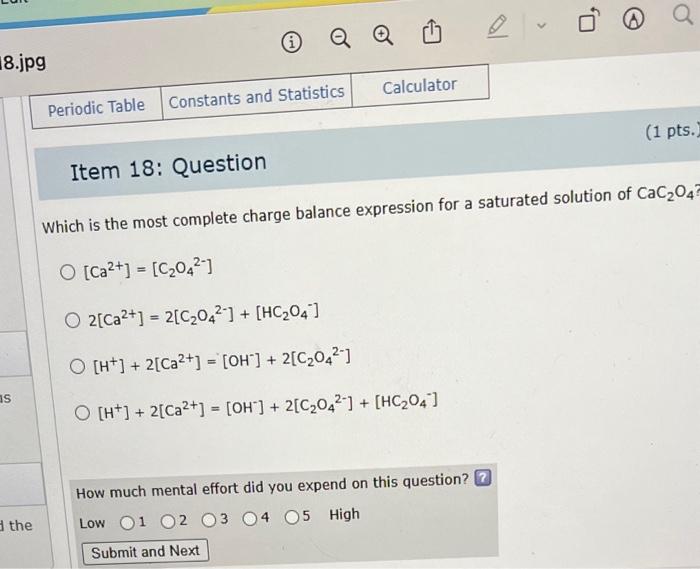
Which is the most complete charge balance expression for a saturated solution of CaC2O4 ? [Ca2+]=[C2O42]2[Ca2+]=2[C2O42]+[HC2O4][H+]+2[Ca2+]=[OH]+2[C2O42][H+]+2[Ca2+]=[OH]+2[C2O42]+[HC2O4] How much mental effort did you expend on this question? \begin{tabular}{l|l|l|l|l|l|l} Low & 1 & 2 & 3 & 4 & 5 & High \end{tabular}
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


