Question: Which of the following will form a buffer solution when added to 100 mL of 1M a NaOH? 1.0.1 mol HCI II. 0.2 mol HF
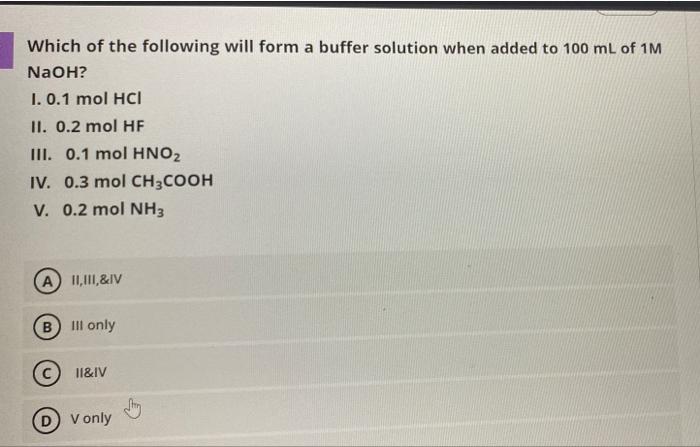
Which of the following will form a buffer solution when added to 100 mL of 1M a NaOH? 1.0.1 mol HCI II. 0.2 mol HF III. 0.1 mol HNO2 IV. 0.3 mol CH3COOH V. 0.2 mol NH3 A 11,111,&IV Bill only 11&IV D V only
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


