Question: which one is correct 11. Choose the CORRECT statement that explains reducing property of methanoic acid with KMnO /H,O*.* On treating methanoic acid with strong
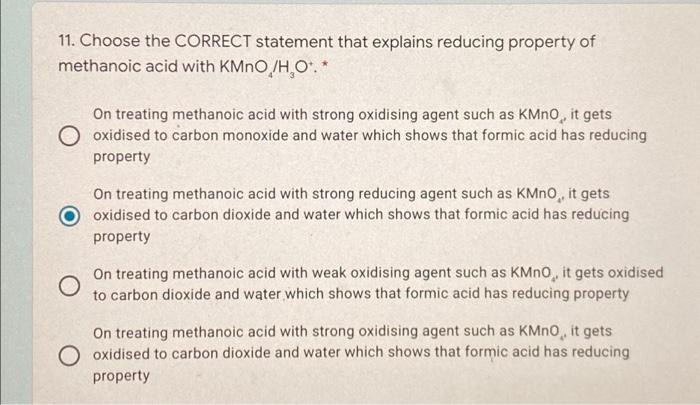
11. Choose the CORRECT statement that explains reducing property of methanoic acid with KMnO /H,O*.* On treating methanoic acid with strong oxidising agent such as KMno, it gets O oxidised to carbon monoxide and water which shows that formic acid has reducing property On treating methanoic acid with strong reducing agent such as KMnO, it gets oxidised to carbon dioxide and water which shows that formic acid has reducing property On treating methanoic acid with weak oxidising agent such as KMnO, it gets oxidised to carbon dioxide and water which shows that formic acid has reducing property On treating methanoic acid with strong oxidising agent such as KMnO, it gets O oxidised to carbon dioxide and water which shows that formic acid has reducing property
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


