Question: Which reaction, 1, 2, or 3 in each group, will be either faster or more likely to succeed in forming significant amounts of desired product.
Which reaction, 1, 2, or 3 in each group, will be either faster or more likely to succeed in forming significant amounts of desired product. Give a brief, but clear and very concise explanation for all your choices.
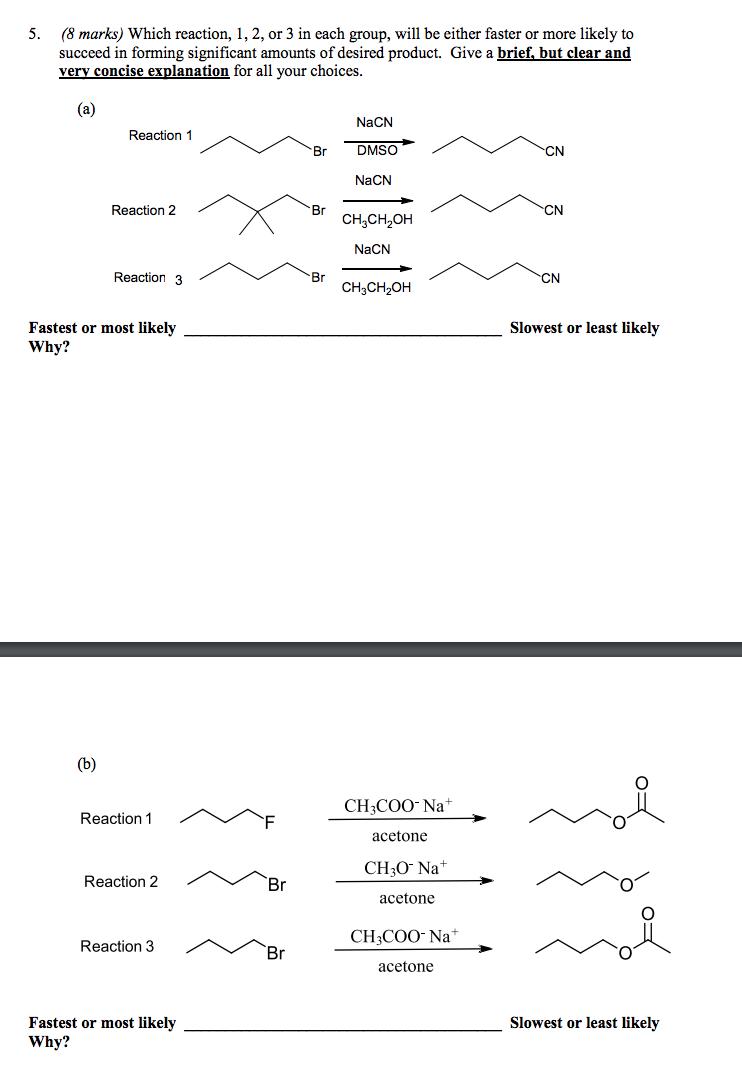
(8 marks) Which reaction, 1, 2, or 3 in each group, will be either faster or more likely to succeed in forming significant amounts of desired product. Give a brief, but clear and very concise explanation for all your choices. 5. (a) NaCN Reaction 1 Br DMSO CN NaCN Reaction 2 Br CH,CH, CN NaCN Reaction 3 Br H,CH, CN Fastest or most likely Slowest or least likely Why? (b) CH3COO- Na+ Reaction 1 acetone CH3O Na+ Reaction 2 Br acetone CH3COO- Na* Reaction 3 Br acetone Fastest or most likely Slowest or least likely Why?
Step by Step Solution
3.39 Rating (158 Votes )
There are 3 Steps involved in it
To determine the fastest or most likely reactions well analyze each group based on solvent effects l... View full answer

Get step-by-step solutions from verified subject matter experts


