Question: Which solid will precipitate first if an aqueous solution of AgNO3 at 25C is slowly added to an aqueous solution containing 0.01 M Na CrO4
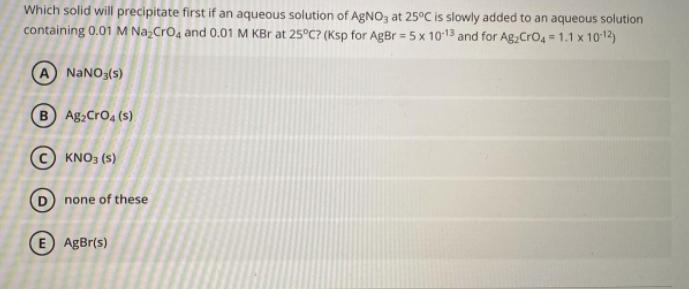
Which solid will precipitate first if an aqueous solution of AgNO3 at 25C is slowly added to an aqueous solution containing 0.01 M Na CrO4 and 0.01 M KBr at 25C? (Ksp for AgBr = 5 x 10-13 and for Ag,CrO 4 = 1.1 x 10-12 NaNO3(s) B Ag2Cro, (s) KNO3 (5) none of these E AgBr(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


