Question: Which statement about pka is not correct? * (1 Point) The pka of an acid = -logka The higher the value of pKa the stronger
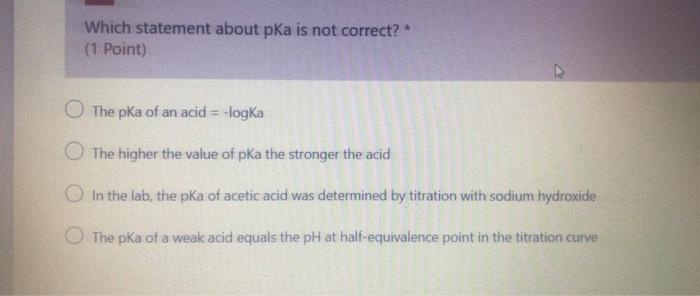
Which statement about pka is not correct? * (1 Point) The pka of an acid = -logka The higher the value of pKa the stronger the acid In the lab, the pKa of acetic acid was determined by titration with sodium hydroxide The pka of a weak acid equals the pH at half-equivalence point in the titration curve
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


