Question: Which statement is not true about boron trifluoride, BF3, below? Boron trifluoride, BF3 a. It has a molecular geometry of trigonal planar. b. The bond
Which statement is not true about boron trifluoride, BF3, below? Boron trifluoride, BF3
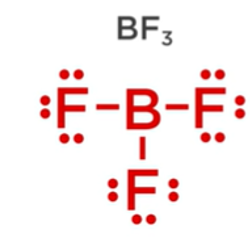
a. It has a molecular geometry of trigonal planar.
b. The bond angles are 120 degrees each.
c. It has an electron domain geometry of trigonal planar.
d. All atoms in the molecule satisfy the octet rule.
Which is most likely the value of the F-Xe-O bond angle?
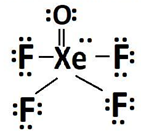
a.
92 degrees
b.
120 degrees
c.
90 degrees
d.
88 degrees
BF3 :- -B-F :F; :0: || .. F-F -Xe :F: :F
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


