Question: While there are many ways to solve this problem, one strategy is to calculate the volume of any metal's unit cell given its theoretical density
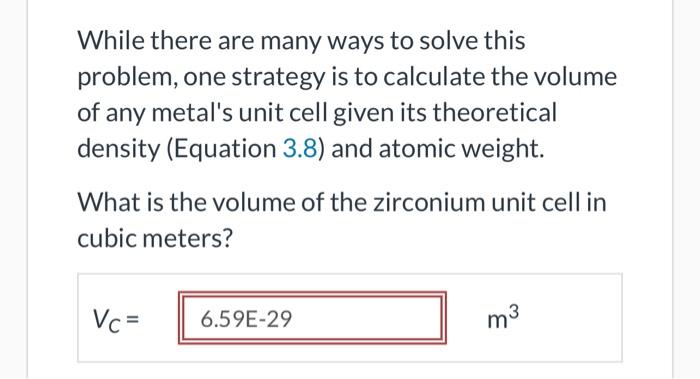
While there are many ways to solve this problem, one strategy is to calculate the volume of any metal's unit cell given its theoretical density (Equation 3.8) and atomic weight. What is the volume of the zirconium unit cell in cubic meters? VC=m3 While there are many ways to solve this problem, one strategy is to calculate the volume of any metal's unit cell given its theoretical density (Equation 3.8) and atomic weight. What is the volume of the zirconium unit cell in cubic meters? VC=m3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


