Question: whoever keeps answering... youre wrong. the numbers have changed. i do not appreciate you continally answering my question wrong. either answer correct or not at
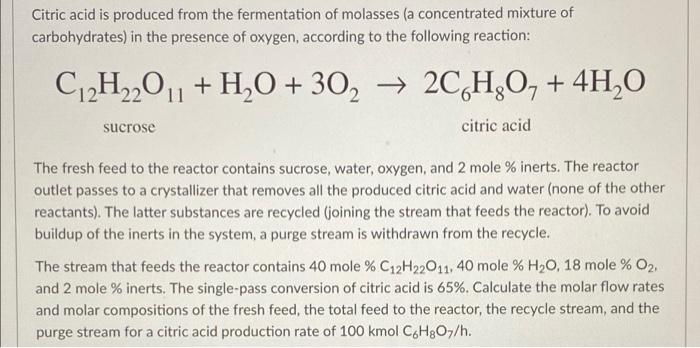
Citric acid is produced from the fermentation of molasses (a concentrated mixture of carbohydrates) in the presence of oxygen, according to the following reaction: C2H22011 + H2O + 302 2C,H,O, + 4H2O sucrose citric acid The fresh feed to the reactor contains sucrose, water, oxygen, and 2 mole % inerts. The reactor outlet passes to a crystallizer that removes all the produced citric acid and water (none of the other reactants). The latter substances are recycled (joining the stream that feeds the reactor). To avoid buildup of the inerts in the system, a purge stream is withdrawn from the recycle. The stream that feeds the reactor contains 40 mole % C12H22011, 40 mole % H20, 18 mole % O2, and 2 mole % inerts. The single-pass conversion of citric acid is 65%. Calculate the molar flow rates and molar compositions of the fresh feed, the total feed to the reactor, the recycle stream, and the purge stream for a citric acid production rate of 100 kmol CH307/h. Citric acid is produced from the fermentation of molasses (a concentrated mixture of carbohydrates) in the presence of oxygen, according to the following reaction: C2H22011 + H2O + 302 2C,H,O, + 4H2O sucrose citric acid The fresh feed to the reactor contains sucrose, water, oxygen, and 2 mole % inerts. The reactor outlet passes to a crystallizer that removes all the produced citric acid and water (none of the other reactants). The latter substances are recycled (joining the stream that feeds the reactor). To avoid buildup of the inerts in the system, a purge stream is withdrawn from the recycle. The stream that feeds the reactor contains 40 mole % C12H22011, 40 mole % H20, 18 mole % O2, and 2 mole % inerts. The single-pass conversion of citric acid is 65%. Calculate the molar flow rates and molar compositions of the fresh feed, the total feed to the reactor, the recycle stream, and the purge stream for a citric acid production rate of 100 kmol CH307/h
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


