Question: Why is it that for the first example you get a mixture of 2 trans products, but in the second example you only get 1
Why is it that for the first example you get a mixture of 2 trans products, but in the second example you only get 1 product when the dienophiles in both examples are trans?
How do I know for Diels Alder Reactions when I will get 2 products instead of 1?
?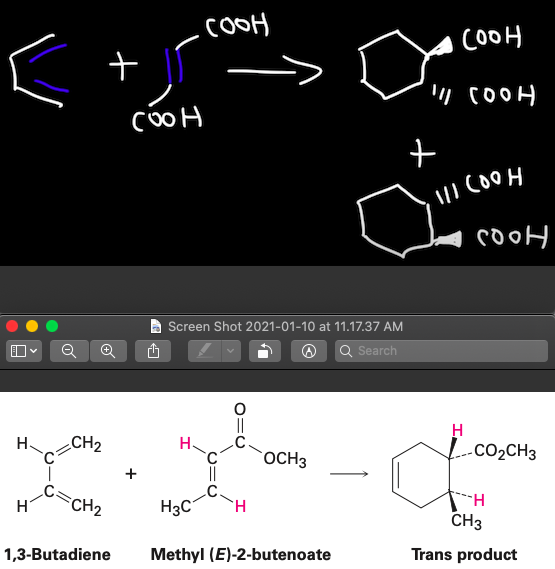
. (0 (+" + ( + ( Screen shot 2021-01-10 at 11.17.37 AM 0 11 Search || . CH2 .co . -CO2CH3 3 + H - CH2 . CH3 1,3-Butadiene Methyl (E)-2-butenoate Trans product
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


