Question: Williamson Ether Synthesis experiment quantities to work from unknown: 3-methylphenoxyacetic acid found from melting point of 102.9 C unknown initial 1g experiment failed it did

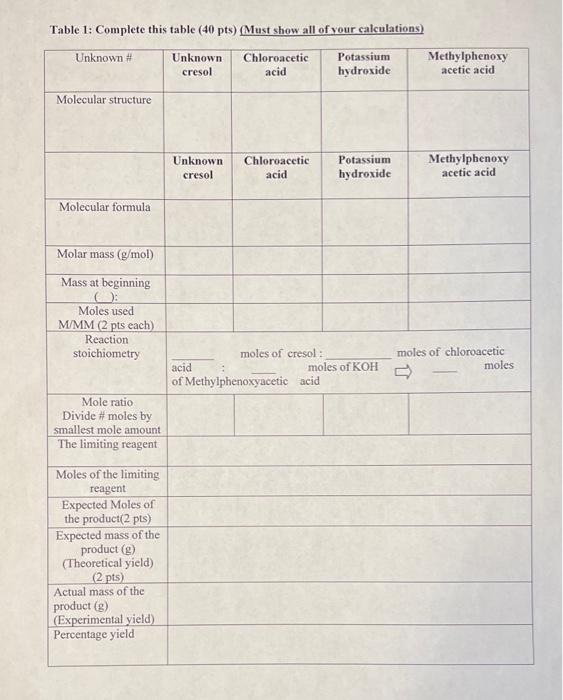
1. What are the literature melting points of the three products chained from three different farmen of cloth, met-and practical) Include the recitation (10) 2 of 4 weite the balanced chemical exation. She reaction you carried out in this experiment alle sue to we structures than molecular formula, timbe new and charge Malone Show de calculation of the old wideo demoty-1.156 g, and volume, and take into consideration of the fact that a 50%. by us solution is wed) Table 1 Complete the table (pt) (Masthan all your calculation Un Caloroacetic wild Possum Hydroxide Methylpheny wete acid Molecular Unknown Chlorometi Potassium hydroxide Methylpheny the acid Molecular Melaras Masining Melessed MMM Gps the Rece stoichiomety miles of de les of cred melose KOH of Medytphonenyacetic acid Molle Dividens by smallest male The Imigrese Mules of the ting Expected Miles of the peadatlap Expected man of the poudact (Thomcticali Actualms of the Experimental de Percentage 1. What are the literature melting points of the three products obtained from three different isomers of cresol (ortho-, meta- and para-cresol). Include the reference citations. (10 pts) 2. Write the balanced chemical equation for the reaction you carried out in this experiment. (Be sure to use structures than molecular formula, reaction must be mass and charge balanced) (7 pts) 3. Show the calculation of the mass of chloroacetic acid used (use density=1.156 g/mL, and volume, and take into consideration of the fact that a 50% by mass solution is used) (8 pts) Table 1: Complete this table (40 pts) (Must show all of your calculations) Unknown Unknown cresol Chloroacetic acid Potassium hydroxide Methylphenoxy acetic acid Molecular structure Unknown cresol Chloroacetic acid Potassium hydroxide Methylphenoxy acetic acid Molecular formula Molar mass (g/mol) Mass at beginning O: Moles used M/MM (2 pts each) Reaction stoichiometry moles of cresol: acid moles of KOH of Methylphenoxyacetic acid moles of chloroacetic moles Mole ratio Divide #moles by smallest mole amount The limiting reagent Moles of the limiting reagent Expected Moles of the product(2 pts) Expected mass of the product() (Theoretical yield) (2 pts) Actual mass of the product (8) (Experimental yield) Percentage yield
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


