Question: With solution and explanation so I can review it. 1. What is the change in entropy of the system if I add 40 J of
With solution and explanation so I can review it.
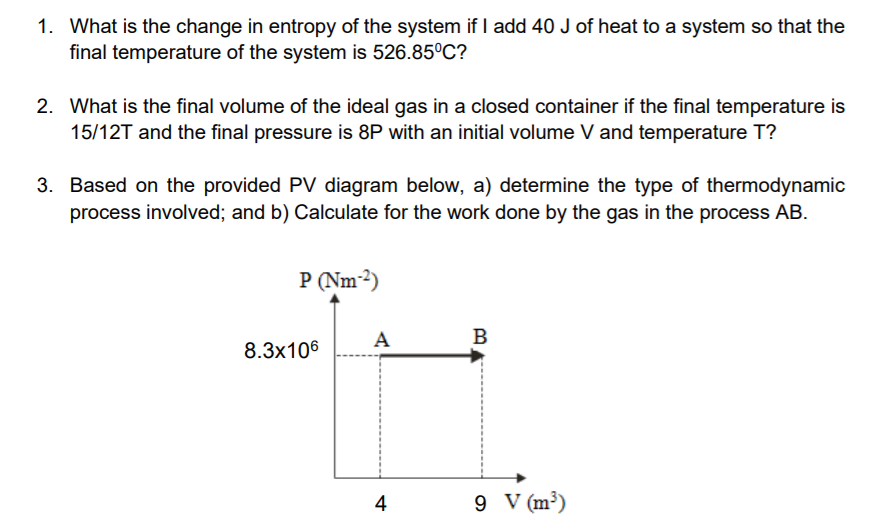
1. What is the change in entropy of the system if I add 40 J of heat to a system so that the final temperature of the system is 526.85C? 2. What is the final volume of the ideal gas in a closed container if the final temperature is 15/12T and the final pressure is 8P with an initial volume V and temperature T? 3. Based on the provided PV diagram below, a) determine the type of thermodynamic process involved; and b) Calculate for the work done by the gas in the process AB. P (Nm-2) 8.3x106 A B 4 9 V (m3)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


