Question: with this information can you answer theses questions? The time is in increments of 30 seconds finish them out and or correct them if needed.
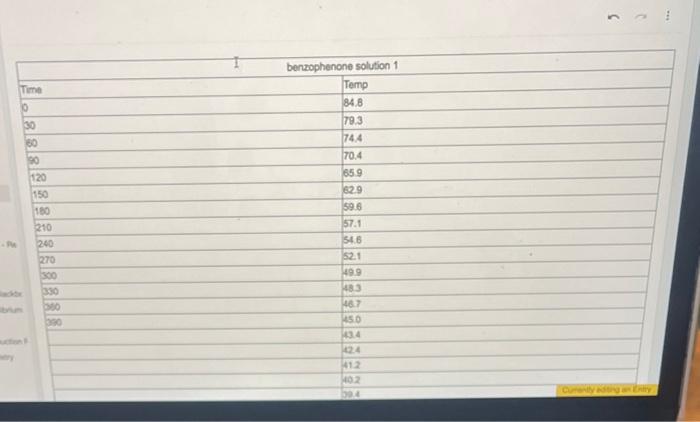
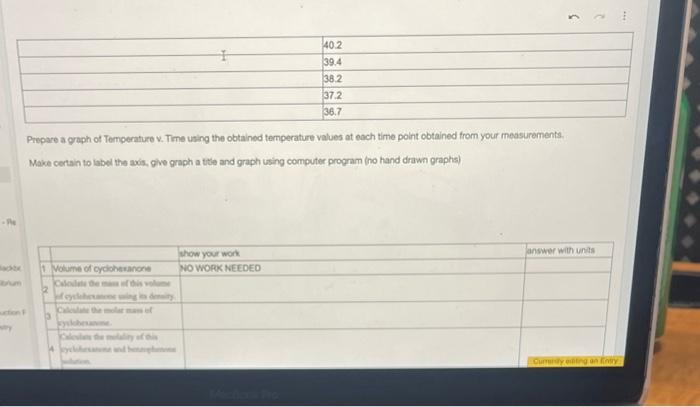
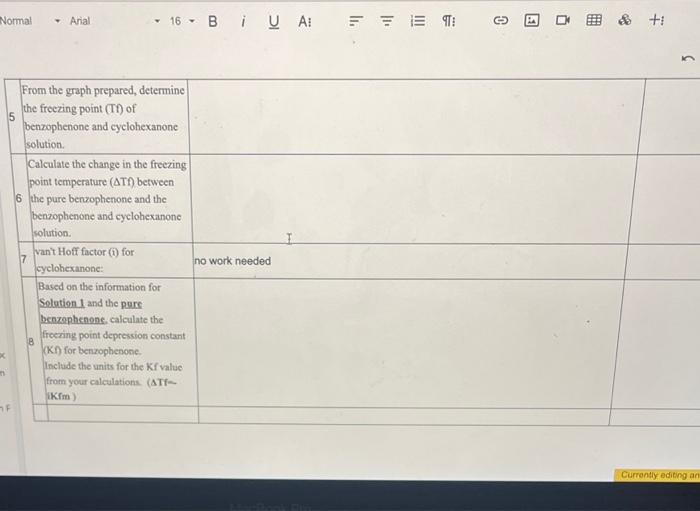
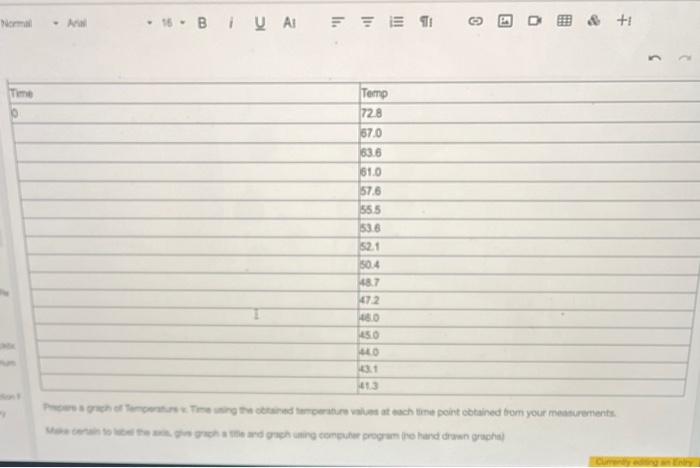
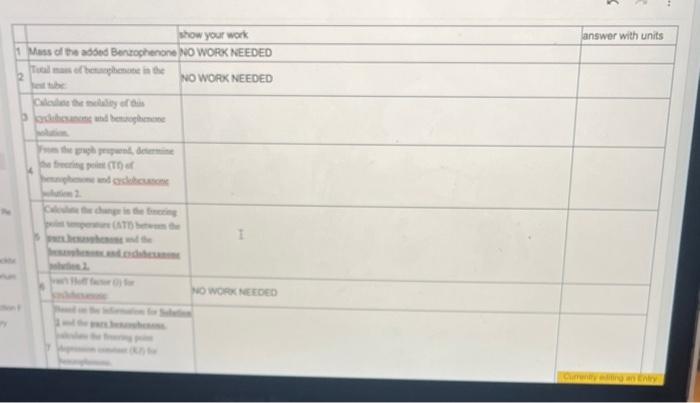

Prepare a graph of Temperature v. Time using the obtained temperature values at each time point obtained from your measurements. Make certain to label the axis, give graph a tile and graph using computer program (no hand drawn graphs) From the graph prepared, determine the freczing point (Tt) of benzophenone and cyclohexanone solution. Calculate the change in the freezing point temperature (Tf) between 6 the pure benzophenone and the benzophenone and cycloheranone solution. 7 van' Hoff factor (i) for cyclohexanone: no work needed Based on the information for Selation 1 and the pore benzophenons, calculate the freecing point depression constant (K I) for benzophenone. Include the units for the Kf value from your calculations ( ATf= IKFm ) \begin{tabular}{|l|l|} \hline Fere & Temp \\ \hline 9 & 72.8 \\ \hline & 67.0 \\ \hline & 63.6 \\ \hline & 61.0 \\ \hline & 57.6 \\ \hline & 55.5 \\ \hline & 53.6 \\ \hline & 52.1 \\ \hline & 50.4 \\ \hline & 48.7 \\ \hline & 47.2 \\ \hline & 46.0 \\ \hline & 45.0 \\ \hline & 440 \\ \hline \end{tabular} Campure te frocriag point Scyreaioe senturas far Soluaion 1 Kf for solution 1: and Solution 2. Kf for solution 2: How do these values compare? Do you erpect these values to be the same? Why or why not? posible sources of enor 7 enor between published 10 and pai S werar betwen published kI and nos. powsile soures of enar? Prepare a graph of Temperature v. Time using the obtained temperature values at each time point obtained from your measurements. Make certain to label the axis, give graph a tile and graph using computer program (no hand drawn graphs) From the graph prepared, determine the freczing point (Tt) of benzophenone and cyclohexanone solution. Calculate the change in the freezing point temperature (Tf) between 6 the pure benzophenone and the benzophenone and cycloheranone solution. 7 van' Hoff factor (i) for cyclohexanone: no work needed Based on the information for Selation 1 and the pore benzophenons, calculate the freecing point depression constant (K I) for benzophenone. Include the units for the Kf value from your calculations ( ATf= IKFm ) \begin{tabular}{|l|l|} \hline Fere & Temp \\ \hline 9 & 72.8 \\ \hline & 67.0 \\ \hline & 63.6 \\ \hline & 61.0 \\ \hline & 57.6 \\ \hline & 55.5 \\ \hline & 53.6 \\ \hline & 52.1 \\ \hline & 50.4 \\ \hline & 48.7 \\ \hline & 47.2 \\ \hline & 46.0 \\ \hline & 45.0 \\ \hline & 440 \\ \hline \end{tabular} Campure te frocriag point Scyreaioe senturas far Soluaion 1 Kf for solution 1: and Solution 2. Kf for solution 2: How do these values compare? Do you erpect these values to be the same? Why or why not? posible sources of enor 7 enor between published 10 and pai S werar betwen published kI and nos. powsile soures of enar
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


