Question: with this problem 2. Consider an experiment in which 45.0 g of diboron hexahydride reacts with 45.0 g of chlorine gas according to the balanced
with this problem
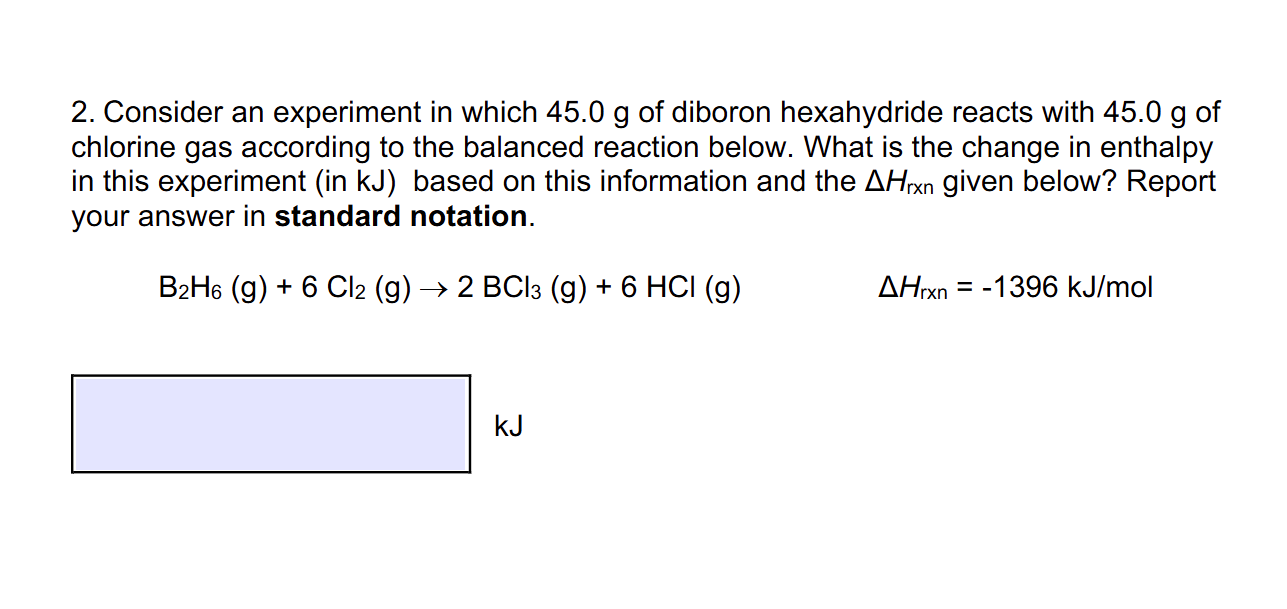 2. Consider an experiment in which 45.0 g of diboron hexahydride reacts with 45.0 g of chlorine gas according to the balanced reaction below. What is the change in enthalpy in this experiment (in kJ) based on this information and the AHrxn given below? Report your answer in standard notation. B2H6 (g) + 6 Cl2 (g) -
2. Consider an experiment in which 45.0 g of diboron hexahydride reacts with 45.0 g of chlorine gas according to the balanced reaction below. What is the change in enthalpy in this experiment (in kJ) based on this information and the AHrxn given below? Report your answer in standard notation. B2H6 (g) + 6 Cl2 (g) -Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


