Question: Without doing any detailed cakculations (but using a periodic table to give atomic weights), rank the following samples in order of increasing number of atoms:
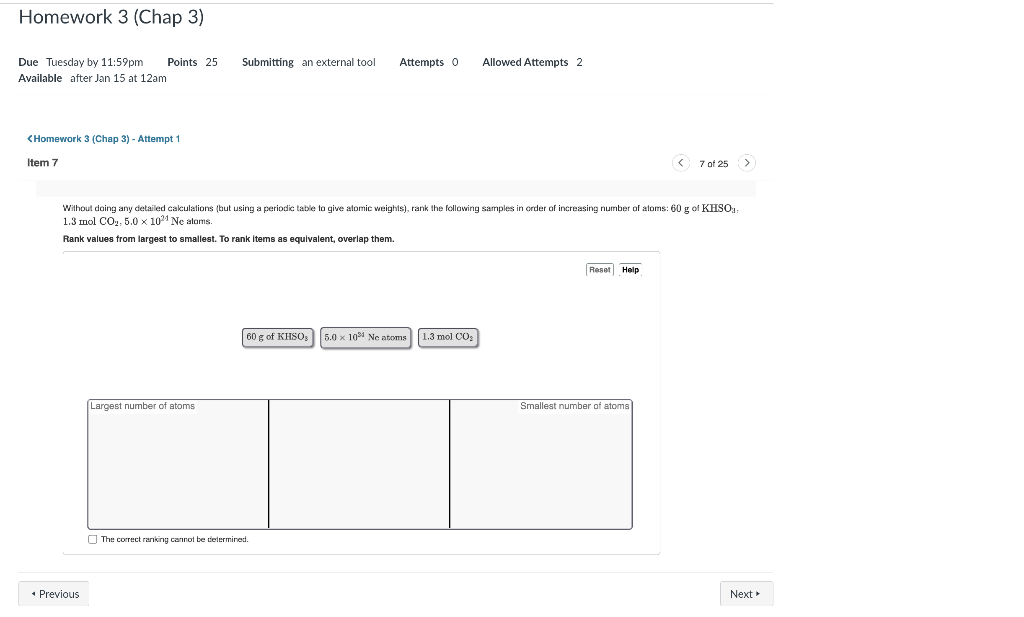
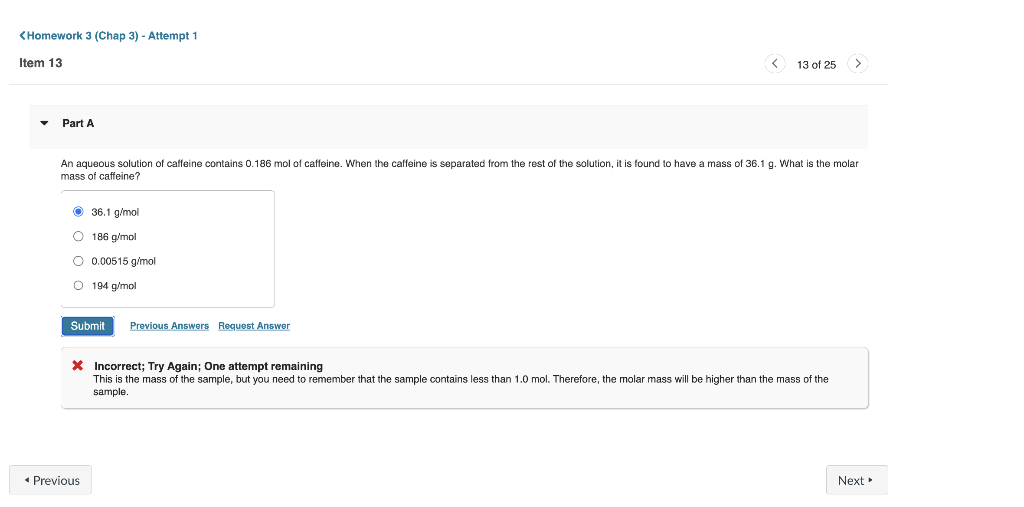
Without doing any detailed cakculations (but using a periodic table to give atomic weights), rank the following samples in order of increasing number of atoms: 60 g of KHSO : 1.3 mol CO2,5.01021Ne atoms. Rank values from largest to smallest. To rank items as equivalent, overlap them. An aqueous solution of caffeine contains 0.186mol of caffeine. When the caffeine is separated from the rest of the solution, it is found to have a mass of 36.1g. What is the molar mass of caffeine? 36.1g/mol 186g/mol 0.00515g/mol 194g/mol * Incorrect; Try Again; One attempt remaining This is the mass of the sample, but you need to remember that the sample contains less than 1.0 mol. Therefore, the molar mass will be higher than the mass of the sample
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


