Question: won will save this one Question 4 At 1000K, the value of Kp for the following reaction equals 0.338 2 S03 (9) 2 SO2 (s)
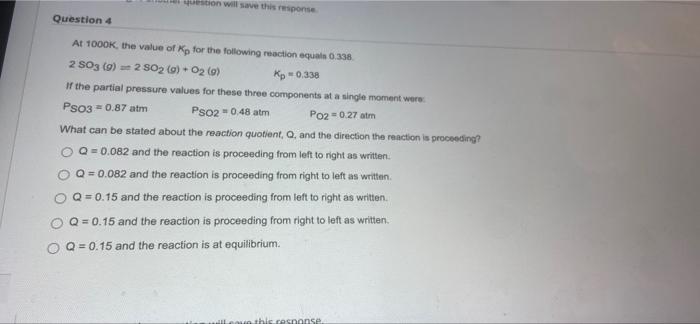
won will save this one Question 4 At 1000K, the value of Kp for the following reaction equals 0.338 2 S03 (9) 2 SO2 (s) + O2(o) Rp 0,338 If the partial pressure values for these three components at a single moment were Pso3 0.87 atm Pso2 * 0.48 atm Po2 = 0.27 am What can be stated about the reaction quotient, Q. and the direction the reaction is proceeding? O Q=0.082 and the reaction is proceeding from left to right as written Q = 0.082 and the reaction is proceeding from right to left as written Q=0.15 and the reaction is proceeding from left to right as written. O Q = 0.15 and the reaction is proceeding from right to left as written Q = 0.15 and the reaction is at equilibrium. noun this rosnanse
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


