Question: Would anyone be able to do this on paper ?? or just in calculations instead of code? = (3) (5 points) The chemical reaction from
 Would anyone be able to do this on paper ?? or just in calculations instead of code?
Would anyone be able to do this on paper ?? or just in calculations instead of code?
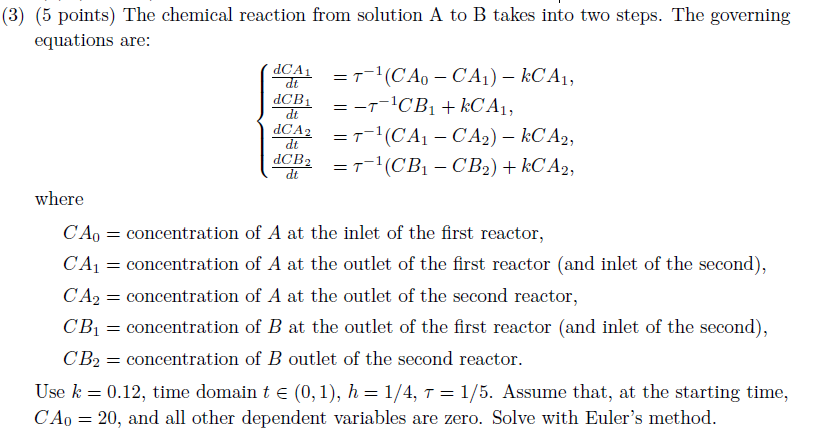
= (3) (5 points) The chemical reaction from solution A to B takes into two steps. The governing equations are: (ACA = 7-1(CA. - CA1) kC A1, dCB1 E-T-CB1 + KC A1, dCA2 = 7-1(CA1 - CA2) kC A2, dCB2 =T-1(CB1 - CB2) + kC A2, dt = dt = dt dt where = = CA = concentration of A at the inlet of the first reactor, CA1 = concentration of A at the outlet of the first reactor (and inlet of the second), CA2 = concentration of A at the outlet of the second reactor, CB1 = concentration of B at the outlet of the first reactor (and inlet of the second), CB2 = concentration of B outlet of the second reactor. Use k = 0.12, time domain t (0,1), h = 1/4, T = 1/5. Assume that, at the starting time, CA0 = 20, and all other dependent variables are zero. Solve with Euler's method. = = =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


