Question: Write a balanced chemical equation for the decomposition reaction. Do not include phases. Part 2 (1 point) x Feedback Calculate the mass of CO2 produced
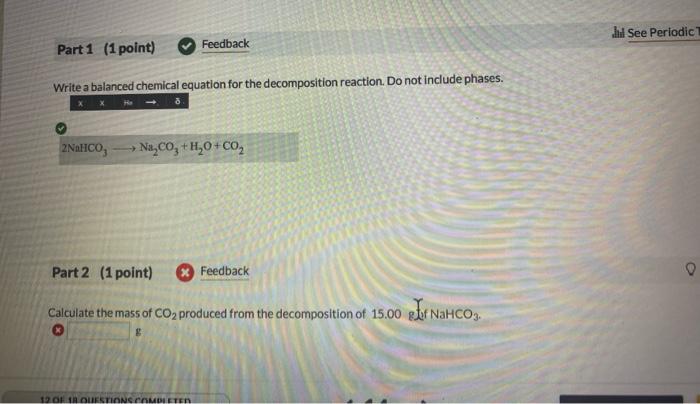
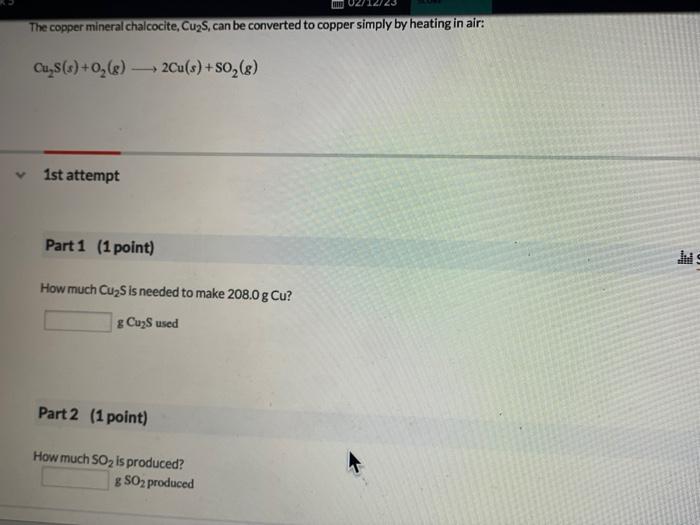
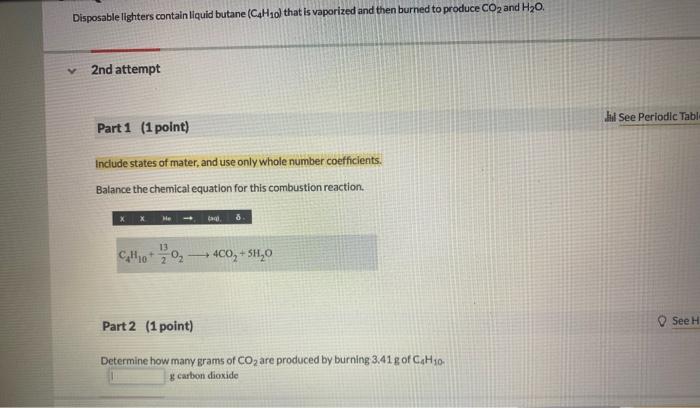
Write a balanced chemical equation for the decomposition reaction. Do not include phases. Part 2 (1 point) x Feedback Calculate the mass of CO2 produced from the decomposition of 15.00 g gef NaHCO3. g The copper mineral chalcocite, Cu2S, can be converted to copper simply by heating in air: Cu2S(s)+O2(g)2Cu(s)+SO2(g) 1st attempt Part 1 (1 point) How much Cu2S is needed to make 208.0gCu ? gCu2S used Disposable lighters contain liquid butane (C4H1 o) that is vaporized and then burned to produce CO2 and H2O. 2nd attempt Part 1 (1 point) Include states of mater, and use only whole number coefficients. Balance the chemical equation for this combustion reaction. C4H10+213O24CO2+5H2O Part 2 (1 point) Determine how many grams of CO2 are produced by burning 3.41g of C4H10 g carbon dioxide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


