Question: Write a MATLAB CODE: The Peng-Robinson equation of state was developed to describe the pressure-volume-temperature (P-V-T) behavior of gasses typically encountered in the petroleum processing
Write a MATLAB CODE:
The Peng-Robinson equation of state was developed to describe the pressure-volume-temperature (P-V-T) behavior of gasses typically encountered in the petroleum processing industry. It was the most popular equation of state in use during the 1970s:
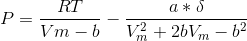
where:
T is the gas temperature, in K (Kelvin)
P is the gas pressure, in atmospheres
Vm is the gas molar volume, in cm3/mol
R is the gas constant; R = 82.06 cm3 atm mol-1 K-1
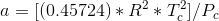
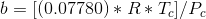
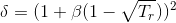
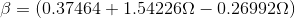
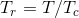
Tc and Pc are the critical temperature and pressure, respectively, for the gas, and ? is the so- called acentric factor.
Using the Peng-Robinson equation of state, write MATLAB code (using a for-end loop) to compute the pressure (P) in atmospheres, of the following gases at T = 800K, using data given in the table. Note that Vm varies over ranges for each gas in the table. Prepare a plot of P vs. Vm, for each gas shown in the table below (i.e., P on the y axis and Vm on the x axis)
| GAS | Pc | Tc(deg) | Vm(cm3/mol) | omega |
| Propane | 48.45 | 375 | 90:5:1000 | 0.153 |
| n-butane | 37.41 | 425 | 500:5:1500 | 0.199 |
| n-heptane | 27.65 | 543 | 750:5:2000 | 0.349 |
NOTE: Again, dont worry about units. They all work out.
RT a* V772-b V, + 2bV,n-b2 m_ m_ P
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


