Question: You are given an unknown ionic solid, which has a van't Hoff factor of i = 2. You complete the the freezing point depression
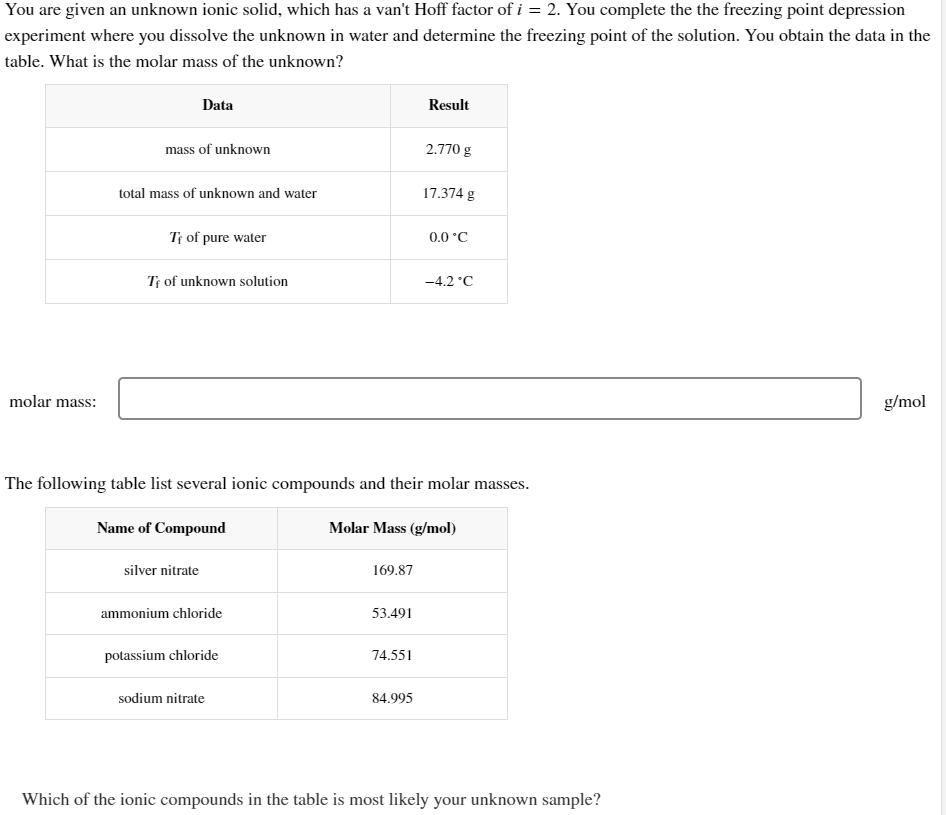
You are given an unknown ionic solid, which has a van't Hoff factor of i = 2. You complete the the freezing point depression experiment where you dissolve the unknown in water and determine the freezing point of the solution. You obtain the data in the table. What is the molar mass of the unknown? Data Result molar mass: mass of unknown 2.770 g total mass of unknown and water 17.374 g T of pure water 0.0C Tf of unknown solution -4.2C The following table list several ionic compounds and their molar masses. Name of Compound silver nitrate Molar Mass (g/mol) 169.87 ammonium chloride 53.491 potassium chloride 74.551 sodium nitrate 84.995 Which of the ionic compounds in the table is most likely your unknown sample? g/mol
Step by Step Solution
There are 3 Steps involved in it
To determine the molar mass of the unknown we can use the freezing point depression formula Delta Tf ... View full answer

Get step-by-step solutions from verified subject matter experts


