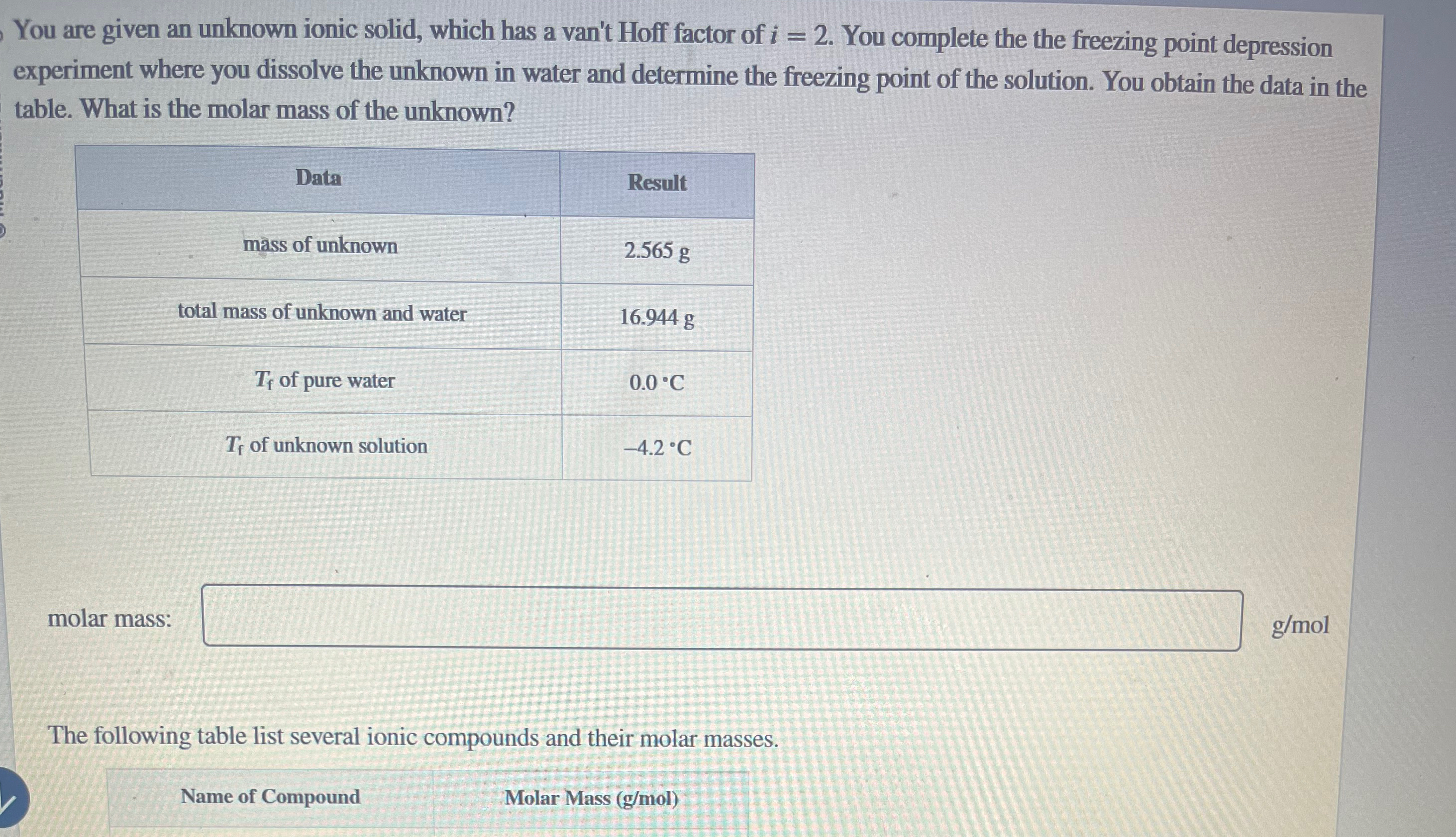
Question: You are given an unknown ionic solid, which has a van't Hoff factor of i = 2 . You complete the the freezing point depression
You are given an unknown ionic solid, which has a van't Hoff factor of You complete the the freezing point depression experiment where you dissolve the unknown in water and determine the freezing point of the solution. You obtain the data in the table. What is the molar mass of the unknown?
tableDataResultmss of unknown,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


