Question: Write equilibrium constant expressions. Write equilibrium constant expressions for the following reactions in terms of concentration: K= (b) MnO2(s)+2H2(g)Mn(s)+2H2O(g) (c) CH3COOH(aq)+H2O()R2H3O+(aq)+CH3COO(aq) K
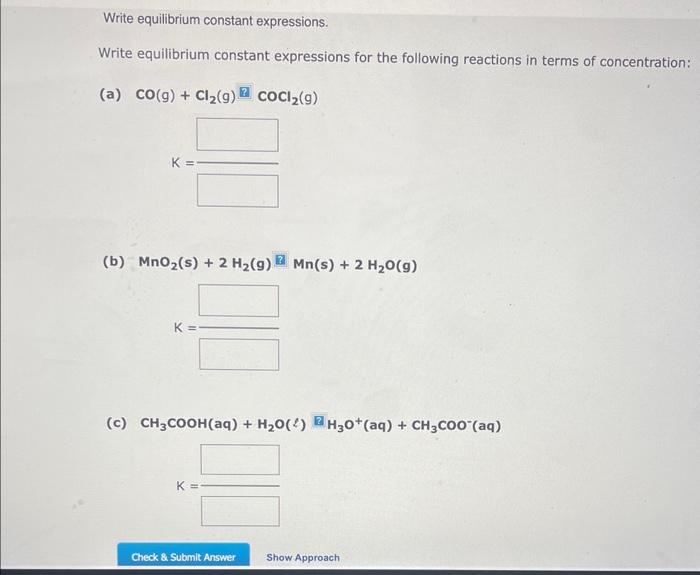
Write equilibrium constant expressions. Write equilibrium constant expressions for the following reactions in terms of concentration: K= (b) MnO2(s)+2H2(g)Mn(s)+2H2O(g) (c) CH3COOH(aq)+H2O()R2H3O+(aq)+CH3COO(aq) K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


