Question: Write equilibrium constant expressions, K c , for the reactions 2 NO2(g) Zn+ Zn+ (aq) + 2 Ag(s) 2+ (a) 2 NO(g) + O2(g) (b)
Write equilibrium constant expressions, Kc, for the reactions
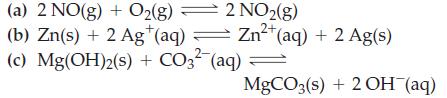
2 NO2(g) Zn+ Zn+ (aq) + 2 Ag(s) 2+ (a) 2 NO(g) + O2(g) (b) Zn(s) + 2 Ag+ (aq) = (c) Mg(OH)2(s) + CO3- (aq) = MgCO3(s) + 2OH(aq)
Step by Step Solution
★★★★★
3.40 Rating (172 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

To write the equilibrium constant expressions Kc for the given reaction... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


