Question: The following reaction begins when 0.56 mol of CO and 0.64 mol of H2O are added to a 2.00 L reaction vessel at a
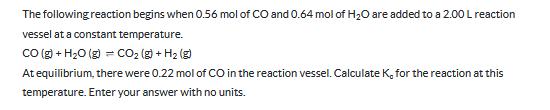
The following reaction begins when 0.56 mol of CO and 0.64 mol of H2O are added to a 2.00 L reaction vessel at a constant temperature. CO(g) + H2O (g) = CO2(g) + H2(g) At equilibrium, there were 0.22 mol of CO in the reaction vessel. Calculate K, for the reaction at this temperature. Enter your answer with no units.
Step by Step Solution
3.49 Rating (162 Votes )
There are 3 Steps involved in it
The balanced chemical equation for the given reaction is COg H2Og CO2g H2g The reaction quotient Qc ... View full answer

Get step-by-step solutions from verified subject matter experts


