Question: Write the balanced NET IONIC equation for the reaction that occurs when hydroiodic acid and barium hypochlorite are combined. Use H3O+instead of H+. This reaction
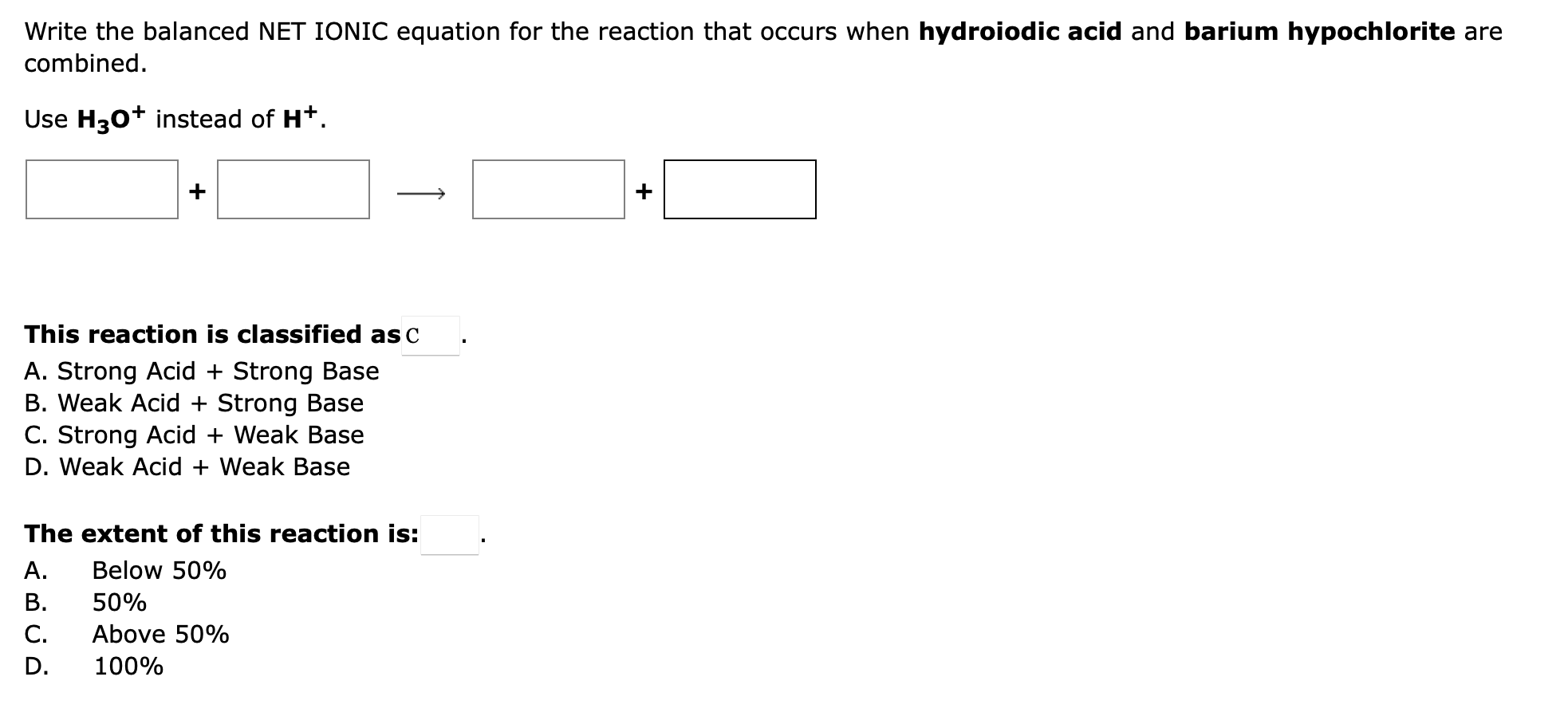
Write the balanced NET IONIC equation for the reaction that occurs when hydroiodic acid and barium hypochlorite are combined. Use H3O+instead of H+. This reaction is classified as A. Strong Acid + Strong Base B. Weak Acid + Strong Base C. Strong Acid + Weak Base D. Weak Acid + Weak Base The extent of this reaction is: A. Below 50\% B. 50% C. Above 50\% D. 100%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


